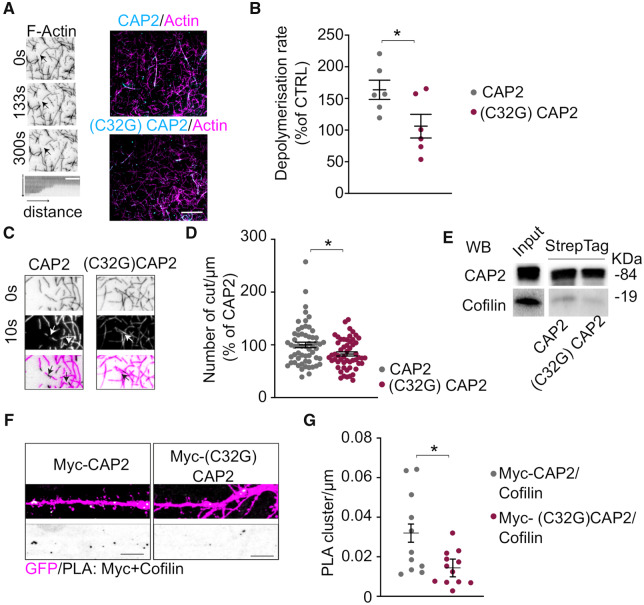
Figure 6.
Cys32-dependent CAP2 dimerization is relevant for association with cofilin and for controlling actin depolymerization. (A) Spontaneous in vitro depolymerization of F-actin-Alexa Fluor 568 visualized by total internal reflection fluorescence microscopy. Left panels, actin filament undergoing depolymerization upon G-actin wash-out, arrowheads indicate the barbed ends of the filament. Right panels, overlay images show the actin filaments (magenta) and either CAP2 or (C32G)CAP2 mutant (cyan). Scale bar = 5 μm. (B) Kymographs analysis of F-actin depolymerization rate indicates that CAP2 increases the depolymerization rate of F-actin while the mutant (C32G)CAP2 abolished this effect [(C32G)CAP2 versus CAP2, unpaired t-test: *P = 0.038, n = 6 independent experiments]. (C) Representative images of the analysis of the severing activity show an actin filament undergoing depolymerization in presence of either CAP2 or (C32G)CAP2 and arrowheads indicate the presence of cutting sites. (D) The number of cuts/μm significantly decreases in presence of (C32G)CAP2 compared to CAP2 [(C32G)CAP2 versus CAP2, unpaired t-test: *P = 0.01, n (C32G)CAP2 = 54 filaments, n CAP2 = 58 filaments]. (E) WB of the Streptag CAP2 and (C32G)CAP2 fusion proteins shows that the mutation of C32 to G reduces CAP2 capability to bind cofilin. Full uncropped blots are available in the supplementary material. (F) Images showing PLA signal between cofilin and either Myc-CAP2 or Myc-(C32G)CAP2 (white) along MAP2 positive dendrites (magenta). Scale bar = 5 μm. Lower panels, inverted images of PLA signal (black). (G) The mutation of Cys32 significantly reduces association with cofilin, as shown in the graphs reporting the quantification of the PLA clusters/μm (Myc-CAP2 = 0.0320 ± 0.005; Myc-(C32G)CAP2 = 0.0144 ± 0.005, unpaired t-test from fixed-effects ordinary least squares regression model: *P = 0.0146, Myc-CAP2 n = 11 neurons, Myc-(C32G)CAP2 n = 12 neurons).