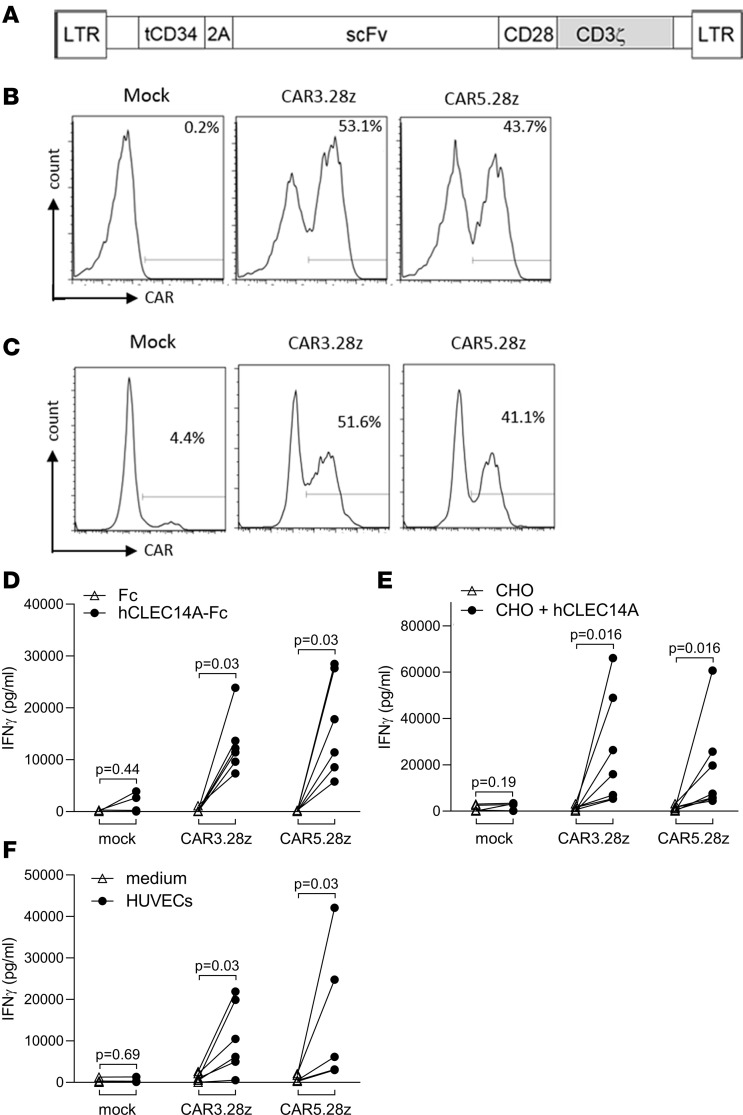
Figure 1. CLEC14A-specific CAR design, expression, and function.
(A) Schematic representation of a recombinant retroviral vector encoding CLEC14A-specific CARs. Retroviral CAR vector (pMP71) coexpresses a truncated CD34 marker gene and an scFv fragment/CD3ζ chain chimeric receptor with a CD28 costimulatory domain. Expression is driven from the LTR promoter, and the 2A peptide linker ensures equimolar expression of both molecules. (B) Recombinant retroviral expression vectors encoding CARs CAR3.28z and CAR5.28z (with scFv fragments from CLEC14A-specific monoclonal antibodies CRT3 or CRT5, respectively) were used to transduce human T cells. CAR expression was detected by staining for the CD34 marker. Percentage values show proportion of cells stained for CD34 compared with mock-transduced T cells. (C) Transduced T cells stained for expression of CAR using CLEC14A-Fc (percentage values show specific binding of CLEC14A-Fc having subtracted background staining with Fc alone). (D–F) Human T cells engineered to express putative CLEC14A-specific CARs (or mock-transduced T cell controls) were tested using an ELISA for IFN-γ production for response to plate-bound recombinant CLEC14A-Fc (extracellular domain) fusion protein (or Fc alone) (D), CHO cells engineered to express full-length human CLEC14A (or CHO transduced with vector alone) (responder/stimulator [R/S] ratio = 6:1) (E), and HUVECs naturally expressing CLEC14A (or medium alone). (R/S ratio = 1:1) (F). In all cases, the different CAR T cell lines were diluted with mock T cells to equalize for transduction efficiency. Cells were stimulated for 18 hours before testing for IFN-γ production. Results of ELISAs show data from 6–7 repeat experiments, having subtracted background responses of T cells alone. All P values shown were calculated using a Wilcoxon matched-pairs signed rank test.