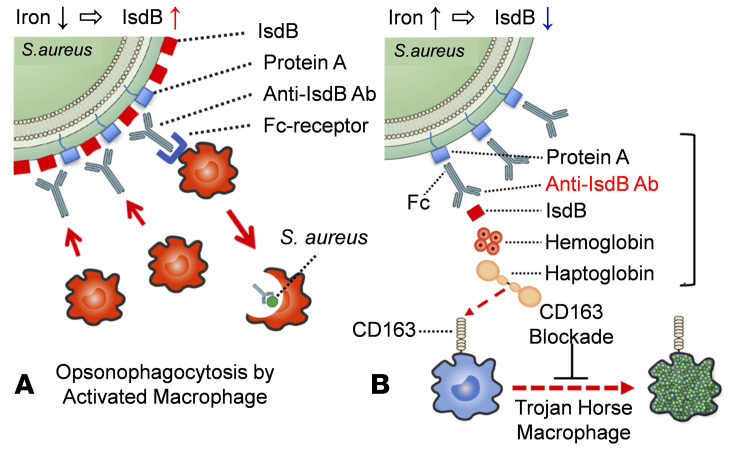
Figure 7. Schematic model of protective versus pathogenic anti-IsdB antibody activity during S. aureus hematogenous versus surgical site infection.
(A) During hematogenous infection, S. aureus responds to the low level of nutritional iron by inducing IsdB production to levels that greatly exceed Spa expression levels, such that anti-IsdB antibody–Fab binding to its antigen on the bacterium is favored. This leads to host protection via opsonophagocytosis by activated macrophages and effective clearance of the infection. (B) In contrast, the high levels of iron at surgical sites that result from bleeding and lysis of red blood cells stimulates the downregulation of IsdB by S. aureus, such that anti-IsdB antibody–Fc binding to Spa is favored over Fab-antigen binding. Subsequent binding by the few shed IsdB molecules to the Fab of the nonneutralizing anti-IsdB antibody and Hb-Hp complex bind to the opsonized IsdB, establishing a multimolecular complex (bracket) that facilitates S. aureus internalization of scavenger macrophages via CD163 receptor–mediated endocytosis. These leukocytes infected with proliferating bacteria act as Trojan horse macrophages to disseminate the S. aureus throughout the host. Based on this theory, drugs that disrupt multimolecular complex formation and/or biological CD163 blockage are predicted to inhibit Trojan horse macrophage formation and prevent sepsis.