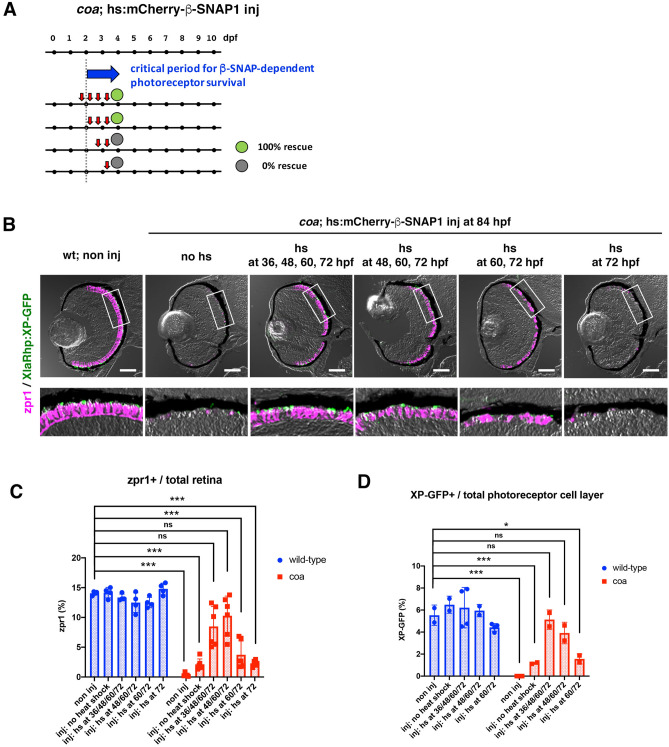
Figure 3.
β-SNAP1 activity after 48 hpf is required for photoreceptor survival at 84 hpf. (A) Experimental design and results of heat-shock promoter-driven β-SNAP1 overexpression in coa mutants. Zebrafish embryos were injected with a DNA construct that expresses mCherry-tagged β-SNAP1 under control of the heat-shock promoter. Heat-shock treatment of injected embryos was performed at 36, 48, 60 and 72 hpf (red arrows), and photoreceptor apoptosis was examined at 84 hpf. Green or grey circles indicate rescue results for cone survival. (B) Retinas of wild-type, non-injected, control embryos and coa mutant embryos injected with a DNA construct encoding hs:mCherry-β-SNAP1 at the one-cell stage and heat-shocked four times at 36/48/60/72 hpf, three times at 48/60/72 hpf, two times at 60/72 hpf, once at 72 hpf, or no heat shock treatment. Cone and rod survival was confirmed by labeling with zpr1 antibody (magenta) and fluorescent signals of Tg[XlaRho:XP-GFP] (green). Three or four heat-shock treatments effectively, and two heat-shock treatments mildly, rescued photoreceptor degeneration in coa mutants. Scale: 50 μm. (C) Percent zpr1-positive area relative to total retinal area. Blue and red bars indicate wild-type and coa mutant embryos injected with DNA encoding hs:mCherry-β-SNAP1. Cone apoptosis was rescued by heat shock treatment at 36/48/60/72 hpf and 48/60/72 hpf, which shows an equivalent rescue level to wild type. Means ± SD. Two-way ANOVA with the Tukey multiple comparison test. ***p < 0.005. (D) Percent XP-GFP-positive area relative to the total area of the photoreceptor cell layer. Blue and red bars indicate wild-type and coa mutant embryos injected with DNA encoding hs:mCherry-β-SNAP1. Rod apoptosis was rescued by heat shock treatment at 36/48/60/72 hpf and 48/60/72 hpf, which shows an equivalent rescue level to wild type. Means ± SD. Two-way ANOVA with Sidak’s multiple comparison test. *p < 0.05, ***p < 0.005.