Figure 5.
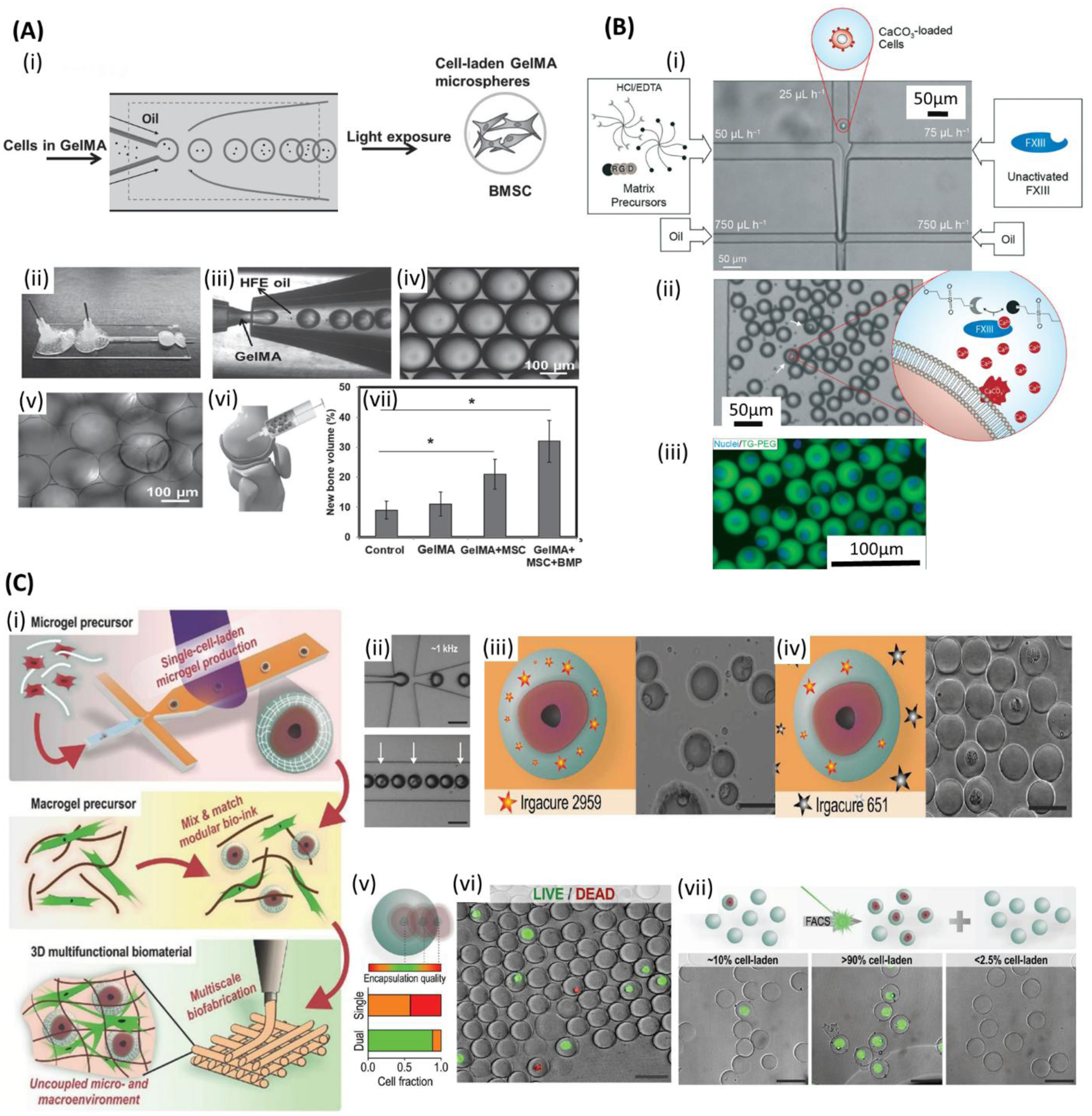
Microfluidic bioprinting of cell-encapsulated microspheres. A) (i) Schematic of bone marrow derived mesenchymal stem cells (BMSCs)-encapsulated gelatin methacryloyl (GelMA) microsphere generation for bone regeneration. (ii) Microfluidic device. (iii) GelMA droplets formation. (iv) Monodisperse GelMA microdroplets. (v) Crosslinked GelMA microspheres. (vi) Implanting the microspheres into the rabbit femoral defect. (vii) New bone volume (%) when implanting normal saline (control) and various contents of microspheres. Reproduced with permission.[299] Copyright 2016, WILEY-VCH Verlag GmbH & Co. B) Selectively gelation of microniches: (i) The process starts with injecting hydrochloric acid and ethylenediaminetetraacetic acid (HCL/EDTA) matrix precursors, diluted solution of CaCo3-loaded cells and inactivated FXII into microfluidic chip, followed by jointing the solution in a laminar flow and shearing the fluid stream with the oily phase, resulting in monodispersed droplets. In the droplets containing CaCo3-loaded cells, HCL dissolve CaCo3 and realise Ca2+ ions that activate FXIII and results in on-demand gelation. (ii) Droplets in the collection channel. (iii) Fluorescent image of the cells (blue: nuclei). Reproduced with permission.[194] Copyright 2017, Royal Society of Chemistry. C) (i) 3D multifunctional biostructures fabrication: Single-cell laden microgel formation followed by modular bioink preparation, and finally 3D bioprinting of multifunctional biomaterials with uncoupled micro- and macroenvironments. (ii) Single cell encapsulation in polyethylene glycol diacrylate (PEGDA) precursor (iii,iv) Schematic and SEM images of failed and prosperous encapsulation using single and dual photoinitiator system, respectively. (v) Cell encapsulation quality regarding the relative position of the encapsulated cells within microgel. (vi) Live/dead staining of encapsulated cells. (green: live; red: dead) (vii) Flow cytometry-based sorting of the cell-laden microgels. Scale bars: (ii-iv, vi,vii) 50 μm. Reproduced with permission.[186] Copyright 2017, WILEY-VCH Verlag GmbH & Co.
