This editorial refers to ‘Mas receptor is translocated to the nucleus upon agonist stimulation in brainstem neurons from spontaneously hypertensive rats but not normotensive rats’ by F.M. Cerniello et al., pp. 1995–2008.
The role of the renin–angiotensin system (RAS) in the pathophysiology of cardiovascular diseases was firmly established more than 60 years ago. Although the RAS was initially defined as a circulating system, works from the 90s confirmed that every organ possesses a local RAS.1 Moreover, intracellular angiotensin (Ang)-II was shown to activate cellular signalling by mechanisms partly independent of plasma membrane receptors, leading to vascular smooth muscle contraction and cell proliferation.2,3 Although these effects were related to Ang receptors located in the cytosol or in endosomes, Ang-II binding sites with nuclear localization were reported as early as 19714 and more recently such receptors were demonstrated to increase nuclear IP3 levels, modulate nucleoplasmic [Ca2+]i and gene transcription.3,5,6
In fact, Ang receptors are not the only G-protein coupled receptors (GPCR) with nuclear localization, many other receptors have been reported to localize at this level, including bradykinin, adrenergic, muscarinic, endothelin, chemokine, and apelin receptors.6,7 In this issue, an elegant study by Cerniello et al.8 demonstrates that the Mas receptor (Mas1R) also translocate to the nucleus after agonist stimulation in brainstem neurons from SHR, but not in normotensive rats. This observation is critical considering that Mas1R is the putative receptor of Ang-(1-7), which is formed under the actions of Ang converting enzyme 2 (ACE2) from Ang-II and exerts vasodilatory and antiproliferative effects, counterbalancing the effects of AT1R activation.9
In addition, Cerniello et al.8 demonstrate that Ang-(1-7) levels and Mas1R expression were enhanced in the nucleus of brainstem neurons from SHR compared to WKY rats, as well as the pool of Mas1R with endosomal localization. In contrast, the recycling of Mas1R from the endosomes back to the plasma membrane was inhibited in SHR neurons. These different trafficking patterns of Mas1R were reflected by a significant reduction in Mas1R functional responses, like arachidonic acid release and phosphorylation of AKT and ERK 1/2, which may contribute to the blunted response to Ang-(1-7) observed in SHR. Furthermore, these data are in line with previous reports showing alterations in the expression of key molecules involved in the intracellular traffic regulation during cardiovascular diseases.6 Yet, some intriguing questions arise from this interesting paper.
There is no doubt that the endosomal pool of receptors results from internalization of plasma membrane receptors and because Mas1R expression is greater in SHR that would also lead to a greater extent of receptors being internalized. Still, the levels of Ang-(1-7) are decreased in this strain and alternative explanations could be that other ligands may induce receptor internalization or that Mas1R undergoes constitutive internalization (i.e. in the absence of the ligand), like in the case of other GPCR.10 In addition, Mas1R was shown to form heterodimers with AT1R and AT2R and might undergo internalization together with these receptors in response to Ang-II.10 Another possibility could be that similar to the effects of ATRAP on AT1R,11 some yet unknown proteins are interacting with Mas1R and promote its endosomal retention. Such a mechanism could also explain the observed diminished Mas1R recycling to the plasma membrane, which may occur concomitantly with a reduced activity of Rab4 and Rab11 GTP-ases, responsible for controlling this trafficking step.
Surprisingly, many GPCR have, embedded in their structure, a nuclear localization signal (NLS). Mas1R has embedded in its C-terminus a monopartite NLS at the positions 284GSSKKKRFKES294 and a bipartite NLS at the positions 288KKRFKESLKVVLTRAFKDEMQPRRQKDN315, motifs which will allow interactions with Ran-GTPase/importin system and its transport from the cytoplasm to nucleus.12 Still, it is not yet clear how endosomal Mas1R can be reached by cytosolic importins, but such traffic from plasma membrane to nucleus via endosomes was reported for ErbB-2 and epidermal growth factor receptor (EGFR).12 Another explanation for the nuclear presence of Mas1R could be direct targeting of newly synthesized receptors in this compartment, although this has not been demonstrated for any member of the GPCR family. Presumably, the functional responses triggered by nuclear receptors will be different from typical plasma membrane Mas1R. Nuclear GPCR were reported to modulate nuclear [Ca2+]i, cAMP levels, H3 histone or ERK1/2 activation and control gene transcription independent of plasma membrane receptors.3,6 These responses can be mediated by nuclear heterotrimeric G-proteins, all the three subunits being detected at this level.6
An unanswered question regarding nuclear Mas1R remains the local source of Ang-(1-7) binding to these receptors. Usually, peptides will be degraded in the endosomes by the local low pH levels, but the authors found that although the nuclear levels of Ang-(1-7) in WKY were negligible, a significant enhancement was observed in SHR after 30 min treatment with extracellular Ang-(1-7). These findings suggest a rapid trafficking pathway of the peptide(s) to nucleus, translocation together with the receptor being the simplest to envision. However, it remains unclear how receptors already bound to the ligand would be able to modulate signalling in the nucleus. Alternatively, nuclear production of ligands might be considered. Cerniello et al.8 found that intracellular levels of Ang-II were increased in SHR, whereas Ang-(1-7) levels were decreased, precluding their traffic to the nucleus together with the internalized GPCR. Yet, measurable Ang-II levels ranging from 5 to 20 fmol/g have been reported in various cells and tissues, including A7r5 rat foetal aortic cell line which is devoid of plasma membrane AT1R and AT2R.3 In addition, even if intracellular production of Ang-II and Ang-(1-7) was never unequivocally demonstrated, the intracellular localization of RAS components was reported by several groups. For instance, Ang converting enzyme (ACE) is internalized in vascular smooth muscle and endothelial cells reaching the nuclear compartment after passing through endosomes.13 More importantly, ACE2, the main enzyme responsible for producing Ang-(1-7) has been found to internalize together with AT1R and this mechanism could explain the low Ang-(1-7) levels in SHR since the enzyme is known to be downregulated in this model.14,15 However, the exact mechanism leading to Ang-(1-7) formation (or of another yet unknown Mas1R endogenous ligand) in the nucleus remains to be characterized, even if the possibility that Mas1R might possess an intrinsic constitutive signalling activity in the nucleus should not be ignored.
Another point highlighted in the present study is the importance of genetic imprinting in the mechanisms of Mas1R internalization and recycling. While primary neuronal cultures are still being criticized, Cerniello et al.8 remind us that neonate neurons are not immature, as already acknowledged by several groups. Interestingly, while SHR do not have hypertension before the 2nd month of life, they already exhibit a modified genetic pool that predisposes them to develop the disease, therefore using neonate neurons from SHR to study the development of hypertension is a highly relevant model. However, some caution is necessary while interpreting data from these experiments. Although SHR neonate neurons already have elevated expression of RAS components, such as ACE and altered electrophysiological properties of neurons within regions involved in cardiovascular regulation, these changes should not be attributed to hypertension since the disease has not yet developed but instead to the genetic imprinting of this model.
Together, Cerniello et al.8 describe a novel mechanism of GPCR regulation (Figure 1) within the RAS, potentially contributing to the development and maintenance of hypertension in SHR. Further work is necessary to clarify the role of Mas1R translocated to the nucleus, the consequences for autonomic regulation and the signalling pathways involved.
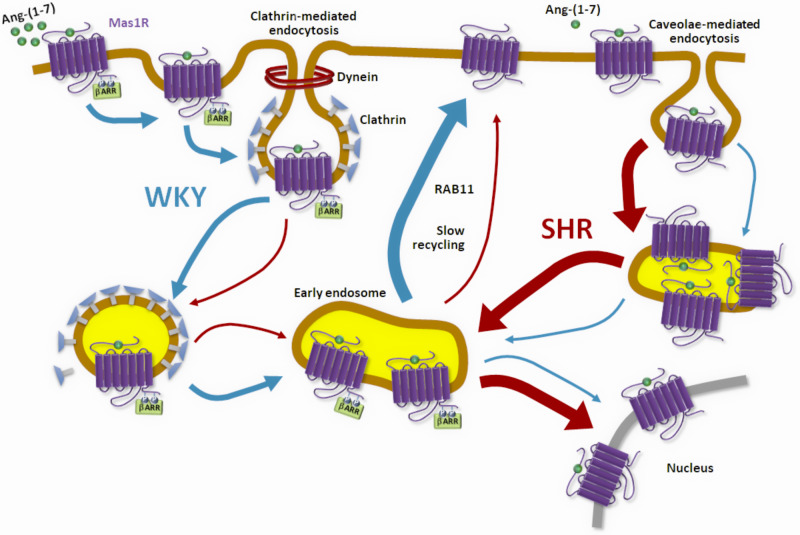
Figure 1.
Mas1R trafficking in hypertension. In normotensive conditions, binding of Ang-(1-7) to Mas1R leads to internalization in early endosomes via clathrin-mediated endocytosis and caveolae-mediated endocytosis followed by a slow recycling to the plasma membrane through Rab11. In hypertension, the caveolae-mediated pathway is up-regulated with enhanced translocation of the receptor to the nucleus and reduced recycling.
Conflict of interest: none declared.
Funding
Funding This work was supported in part by research grants from the National Institutes of Health (HL150592) and the department of Veterans Affairs (BX004294) to C.M.F. and E.L.
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
References
- 1. Lavoie JL, Sigmund CD.. Minireview: overview of the renin-angiotensin system—an endocrine and paracrine system. Endocrinology 2003;144:2179–2183. [DOI] [PubMed] [Google Scholar]
- 2. Haller H, Lindschau C, Erdmann B, Quass P, Luft FC.. Effects of intracellular angiotensin II in vascular smooth muscle cells. Circ Res 1996;79:765–772. [DOI] [PubMed] [Google Scholar]
- 3. Filipeanu CM, Henning RH, Nelemans SA, de Zeeuw D.. Intracellular angiotensin II: from myth to reality? J Renin Angiotensin Aldosterone Syst 2001;2:219–226. [DOI] [PubMed] [Google Scholar]
- 4. Robertson AL Jr, Khairallah PA.. Angiotensin II: rapid localization in nuclei of smooth and cardiac muscle. Science 1971;172:1138–1139. [DOI] [PubMed] [Google Scholar]
- 5. Subedi KP, Paudel O, Sham JS.. Detection of differentially regulated subsarcolemmal calcium signals activated by vasoactive agonists in rat pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 2014;306:C659–C669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tadevosyan A, Vaniotis G, Allen BG, Hébert TE, Nattel S.. G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. J Physiol 2012;590:1313–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Branco AF, Allen BG.. G protein-coupled receptor signaling in cardiac nuclear membranes. J Cardiovasc Pharmacol 2015;65:101–109. [DOI] [PubMed] [Google Scholar]
- 8. Cerniello FM, Silva MG, Carretero OA, Gironacci MM.. Mas receptor is translocated to the nucleus upon agonist stimulation in brainstem neurons from spontaneously hypertensive rats but not normotensive rats. Cardiovasc Res 2020;116:1995–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu P, Sriramula S, Lazartigues E.. ACE2/Ang-(1-7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol 2011; 300:R804–R817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ward RJ, Xu TR, Milligan G.. GPCR oligomerization and receptor trafficking. Methods Enzymol 2013;521:69–90. [DOI] [PubMed] [Google Scholar]
- 11. Lopez-Ilasaca M, Liu X, Tamura K, Dzau VJ.. The angiotensin II type I receptor-associated protein, ATRAP, is a transmembrane protein and a modulator of angiotensin II signaling. Mol Biol Cell 2003;14:5038–5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah P, Chaumet A, Royle SJ, Bard FA.. The NAE pathway: autobahn to the nucleus for cell surface receptors. Cells 2019;8:E915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lucero HA, Kintsurashvili E, Marketou ME, Gavras H.. Cell signaling, internalization, and nuclear localization of the angiotensin converting enzyme in smooth muscle and endothelial cells. J Biol Chem 2010;285:5555–5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM.. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension 2014;64:1368–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK.. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension 2007;49:926–931. [DOI] [PubMed] [Google Scholar]