Abstract
Pathogenic Leptospira spp. are the causative agents of the waterborne zoonotic disease leptospirosis. Leptospira are challenged by numerous adverse conditions, including deadly reactive oxygen species (ROS), when infecting their hosts. Withstanding ROS produced by the host innate immunity is an important strategy evolved by pathogenic Leptospira for persisting in and colonizing hosts. In L. interrogans, genes encoding defenses against ROS are repressed by the peroxide stress regulator, PerR. In this study, RNA sequencing was performed to characterize both the L. interrogans response to low and high concentrations of hydrogen peroxide and the PerR regulon. We showed that Leptospira solicit three main peroxidase machineries (catalase, cytochrome C peroxidase and peroxiredoxin) and heme to detoxify oxidants produced during peroxide stress. In addition, canonical molecular chaperones of the heat shock response and DNA repair proteins from the SOS response were required for Leptospira recovering from oxidative damage. Identification of the PerR regulon upon exposure to H2O2 allowed to define the contribution of this regulator in the oxidative stress response. This study has revealed a PerR-independent regulatory network involving other transcriptional regulators, two-component systems and sigma factors as well as non-coding RNAs that putatively orchestrate, in concert with PerR, the oxidative stress response. We have shown that PerR-regulated genes encoding a TonB-dependent transporter and a two-component system (VicKR) are involved in Leptospira tolerance to superoxide. This could represent the first defense mechanism against superoxide in L. interrogans, a bacterium lacking canonical superoxide dismutase. Our findings provide an insight into the mechanisms required by pathogenic Leptospira to overcome oxidative damage during infection-related conditions. This will participate in framing future hypothesis-driven studies to identify and decipher novel virulence mechanisms in this life-threatening pathogen.
Author summary
Leptospirosis is a zoonotic infectious disease responsible for over one million of severe cases and 60 000 fatalities annually worldwide. This neglected and emerging disease has a worldwide distribution, but it mostly affects populations from developing countries in sub-tropical areas. The causative agents of leptospirosis are pathogenic bacterial Leptospira spp. There is a considerable deficit in our knowledge of these atypical bacteria, including their virulence mechanisms. During infection, Leptospira are confronted with the deadly oxidants produced by the host tissues and immune response. Here, we have identified the leptospiral factors necessary for overcoming infection-related oxidative stress. We found that Leptospira solicit peroxidases to detoxify oxidants as well as chaperones of the heat shock response and DNA repair proteins of the SOS response to recover from oxidative damage. Moreover, our study indicates that the oxidative stress response is orchestrated by a regulatory network involving PerR and other transcriptional regulators, sigma factors, two component systems, and putative non-coding RNAs. These findings provide insights into the mechanisms required by pathogenic Leptospira to tolerate infection-related oxidants and could help identifying novel virulence factors and developing new therapeutic targets.
Introduction
In order to invade a host and establish persistent colonization, pathogens have evolved a variety of strategies to resist, circumvent, or counteract host defenses. Pathogens synthesize enzymes or molecules to eliminate host-produced bactericidal compounds, secrete effectors inhibiting or subverting the host innate immunity, or form biofilms enabling resistance to host defenses.
The strategies used by pathogenic Leptospira for successful host colonization and virulence are not fully understood. These aerobic Gram-negative bacteria of the spirochetal phylum are the causative agents of leptospirosis, a widespread zoonosis [1]. Although recognized as a health threat among impoverished populations in developing countries and tropical areas [2], reported cases of leptospirosis are also on the rise in developed countries under temperate climates [3]. Rodents are the main reservoir for leptospires as the bacteria asymptomatically colonize the proximal renal tubules of these mammals. Infected animals shed bacteria in the environment by their urine and leptospires are transmitted to other animals and humans mostly by exposure to contaminated soils and water. Leptospira penetrate mucous membranes or abraded skin, enter the bloodstream and rapidly disseminate to multiple tissues and organs including kidney, liver and lungs. Clinical manifestations range from a mild flu-like febrile state to more severe and fatal cases leading to hemorrhages and multiple organ failure. The lack of efficient tools and techniques for genetic manipulation of Leptospira spp. and their fastidious growth in laboratory conditions have greatly hampered and limited our understanding of their mechanisms of pathogenicity and virulence [4,5].
As part of the host innate immunity response, reactive oxygen species (ROS), i.e. superoxide anion (•O2-), hydrogen peroxide, (H2O2), hydroxyl radicals (•OH), hypochlorous acid (HOCl), and nitric oxide anion (•NO) are produced upon infection by Leptospira. Indeed, the internalization of pathogenic Leptospira by macrophages and concomitant production of these oxidants have been demonstrated in vitro [6], and leptospirosis-associated oxidative stress has been observed in leptospirosis patients [7] and infected animals [8]. Consistent with these findings was the demonstration that catalase, which catalyzes the degradation of H2O2, is required for Leptospira interrogans virulence [9].
Pathogenic Leptospira spp. are among the rare examples of Gram-negative bacteria in which defenses against peroxide stress, such as catalase, are controlled by a peroxide stress regulator (PerR) and not by OxyR [10]. PerR is a peroxide-sensing transcriptional repressor that belongs to the Fur (Ferric uptake regulator) family of regulators, mostly present in Gram-positive bacteria [11]. The B. subtilis PerR is in a DNA-binding prone conformation in the presence of a regulatory metal (Fe2+) [12]. Upon oxidation by H2O2, PerR releases its regulatory metal and switches to a conformation that cannot bind DNA, leading to the alleviation of gene repression [13,14].
We have conducted a structural and functional characterization of PerR in L. interrogans and showed that Leptospira PerR exhibits the typical metal-induced conformational switch controlling DNA binding and release [15]. Our findings indicated that not only does Leptospira PerR repress defenses against H2O2, but a perR mutant also had a decreased fitness in other host-related stress conditions including in the presence of superoxide [15]. Interestingly, it was shown that perR is up-regulated when Leptospira are exposed in vitro to hydrogen peroxide [15] as well as when Leptospira are cultivated in vivo using Dialysis Membrane Chambers (DMCs) in rats [16], which strongly suggests a role of PerR in the adaptation of pathogenic Leptospira to a mammalian host.
In order to identify the mechanisms solicited by pathogenic Leptospira to adapt to oxidative stress, we determined the global transcriptional response of L. interrogans to H2O2 and assessed the role of PerR in this response. This has revealed the leptospiral factors constituting the first-line of defense against the ROS that Leptospira might encounter when infecting a mammalian host. In addition, our study has identified repair mechanisms allowing leptospires to recover from oxidative damage. Putative regulatory non-coding RNAs were also pinpointed, indicating the complexity of the regulatory network controlling the response to peroxide. We have also identified novel PerR-regulated factors involved in Leptospira survival in the presence of superoxide and assessed their role in Leptospira virulence.
Results
Leptospira transcriptional response to a sublethal concentration of hydrogen peroxide
In order to characterize the transcriptional response of pathogenic Leptospira to hydrogen peroxide, we exposed exponentially growing L. interrogans cells to a sublethal concentration of this oxidant. A 30 min treatment with 10 μM H2O2 (in the presence of iron) was chosen during pilot experiments as having no significant effect on Leptospira viability and growth during logarithmic phase while increasing expression of H2O2-responsive genes such as perR [15]. RNA-Seq (RNA sequencing) was performed to assess RNA abundance and comparison with untreated cells identified a total of 21 genes with differential transcript abundance (see S1 Table for complete data set). Among those, only 13 and 1 genes were respectively up- and down-regulated by at least two-fold with p-values ≤0.05 (See Table 1).
Table 1. Differentially expressed genes upon exposure to sublethal dose of H2O2.
| ORF IDa | Gene | Function | COGsb | Log2FC | Adjusted p-value | FC (RT-qPCR)c |
|---|---|---|---|---|---|---|
| Up-regulated genes | ||||||
| LIMLP_02795 (LIC12927/LA0666) | ccp | Cytochrome C peroxidase | P | 4.764* | 5.31e-43 | 38.900 |
| LIMLP_05955 (LIC11219/LA2809) | ahpC | Peroxiredoxin/alkylperoxiredoxin reductase | O | 3.145* | 3.63e-20 | 11.742 |
| LIMLP_05960 (LIC11220/LA2808) | sufB | Fe-S cluster assembly protein | O | 1.056* | 1.60e-08 | 1.880 |
| LIMLP_10145 (LIC12032/LA1859) | katE | Catalase | P | 1.786 | 2.11e-08 | 3.477 |
| LIMLP_10150 (LIC12033/LA1858) | Ankyrin repeat-containing protein | S | 2.051* | 2.30e-11 | 4.183 | |
| LIMLP_10155 (LIC12034/LA1857) | perR | Regulator Fur familly | T | 2.319* | 1.02e-39 | 6.827 |
| LIMLP_17840 (LIC20008/LB010) | hemA | Glutamyl-tRNA reductase | H | 1.771* | 1.16e-10 | 3.389 |
| LIMLP_17845 (LIC20009/ LB011) | hemC/D | Porphobilinogen deaminase | H | 1.617* | 6.57e-13 | 2.328 |
| LIMLP_17850 (LIC20010/ LB012) | hemB | Delta-aminolevulinic acid dehydratase | H | 1.455* | 2.65e-14 | 2.064 |
| LIMLP_17855 (LIC20011/ LB013) | hemL | Glutamate-1 semialdehyde aminotransferase | H | 1.262* | 1.19e-07 | 2.193 |
| LIMLP_17860 (LIC20012/ LB014) | Signal transduction histidine kinase | T | 1.035* | 2.67e-03 | 2.470 | |
| LIMLP_17865 (LIC20013/ LB015) | Response regulator CheY | K | 1.166* | 1.01e-03 | 2.012 | |
| LIMLP_17870 (LIC20014/ LB016) | hemE | Uroporphyrinogen decarboxylase | H | 1.033* | 1.77e-02 | 2.059 |
| Down-regulated genes | ||||||
| LIMLP_18600 (LIC20149/ LB187) | Permease of the Major facilitator superfamily | P | -1.001 | 1.17e-04 | 0.894 |
Significantly up-and down-regulated genes upon a 30 min exposure to 10 μM H2O2 with a Log2FC cutoff of ± 1 and an adjusted p-value cutoff of 0.05.
a Gene numeration is according to Satou et al. [17]. Corresponding genes of L. interrogans serovar lai strain 56601 and serovar Copenhageni strain Fiocruz L1-130 are indicated in parenthesis.
b The COG functional categories are H, coenzyme transport and metabolism; K, transcription; O, posttranslational modification, protein turnover, chaperones; P, inorganic ion transport and metabolism; S, function unknown; T, signal transduction and metabolism.
c Fold change in gene expression upon a 30 min exposure to 10 μM H2O2 obtained by RT-qPCR experiments.
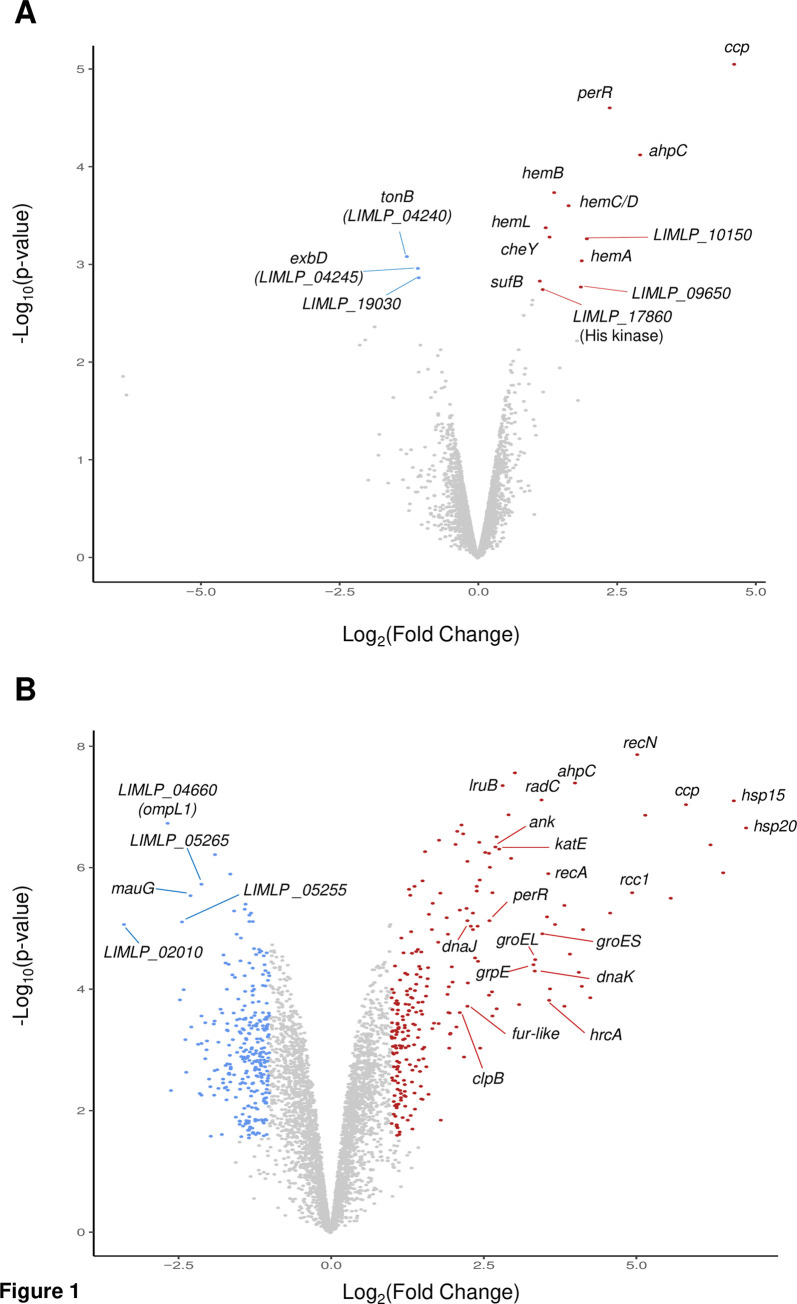
* Genes significantly up-and down-regulated by Volcano analysis (Log2FC cutoff of 1 and p-value cutoff of 0.05 as seen in Fig 1A).
Under a low concentration of H2O2, LIMLP_10145, encoding a catalase, and LIMLP_02795 and LIMLP_05955, coding respectively for a cytochrome C peroxidase and for a peroxiredoxin, were up-regulated with a Log2FC (Log2 Fold Change) of 1.79, 4.76 and 3.14 respectively.
The catalase encoded by LIMLP_10145 (katE) is a monofunctional heme-containing hydroperoxidase, whose catalase activity and periplasmic localization were experimentally demonstrated in pathogenic Leptospira [9,18,19]. The immediate upstream ORF (LIMLP_10150), encoding an ankyrin repeat-containing protein, was also up-regulated with a comparable fold value. In bacteria such as Pseudomonas aeruginosa and Campylobacter jejuni, a protein with ankyrin repeats were found to be required for catalase activity, probably by allowing heme binding [20,21]. In L. interrogans, katE and ank were organized as an operon (S1 Fig) and significant up-regulation of the ank-katE operon upon exposure to sublethal dose of H2O2 was confirmed by RT-qPCR (Table 1 and S1 Fig).
The significantly up-regulated ahpC gene (LIMLP_05955) encodes a peroxiredoxin that reduces H2O2 and tert-Butyl hydroxyperoxide [22]. The SufB-encoding LIMLP_05960 located in the vicinity of ahpC was also up-regulated with a 2-fold. SufB encodes a polypeptide involved in Fe-S cluster assembly proteins. In bacteria such as Escherichia coli, SufB is part of a complex composed of SufB, SufD and the SufC ATPase. SufB is normally found in an operon with sufC and sufD as well as with the other factors of the Suf machinery, i. e. sufE and sufS. L. interrogans genome contains a putative suf cluster (LIMLP_14560–14580 ORFs) and SufE (encoded by LIMLP_05090) but none of the other suf ORFs were significantly regulated by sublethal dose of H2O2. The SufB-encoding LIMLP_05960 shares 40% and 47% identity with SufB from E. coli and B. subtilis, respectively, and most importantly it does contain the critical cysteine residue suggesting that the isolated LIMLP_05960 encodes a bona fide SufB participating in the SufBC2D complex of Fe-S cluster biogenesis. A function or cooperation of SufB with AhpC in H2O2 detoxification remains to be demonstrated.
LIMLP_02795 was another peroxidase-encoding ORF that was greatly up-regulated in the presence of H2O2. LIMLP_02795 encodes a putative Cytochrome C Peroxidase (CCP) that catalyzes the reduction of H2O2 into H2O using the ferrocytochrome as an electron donor. In several L. interrogans genomes, this ORF is annotated as a MauG, a class of Cytochrome C Peroxidase that catalyzes the oxidation of methylamine dehydrogenase (MADH) into tryptophan tryptophylquinone (TTQ) in the methylamine metabolism pathway. LIMLP_02795 exhibits two heme domains with the conventional heme binding motif CXXCH that exists in both CCP and MauG proteins, but it lacks the tyrosine axial ligand for heme (Tyr294 in Paracoccus denitrificans, [23]) that is conserved in all MauGs but replaced by a methionine or histidine residue in CCPs. Therefore, it is very likely that LIMLP_02795 encodes a CCP with a peroxidase activity that is not involved in the methylamine metabolism pathway.
In addition to these three peroxidases, whose increased expression was confirmed by RT-qPCR (Table 1 and S1 and S2 Figs), several ORFs encoding components of heme biosynthesis (LIMLP_17840–17865) were up-regulated by 2 to 3.4-fold (Table 1). Leptospira, unlike other spirochetes, possess a complete heme biosynthesis functional pathway [24]. The ORFs encoding the glutamyl-tRNA reductase (hemA), porphobilinogen deaminase (hemC/D), delta-aminolevulinic acid dehydratase (hemB), glutamate-1 semialdehyde aminotransferase (hemL), uroporphyrinogen-III decarboxylase (hemE), coproporphyrinogen-III oxidase (hemN/F), as well as a two-component system (TCS) (LIMLP_17860 and LIMLP_17865) were organized as an operon (S3 Fig). RT-qPCR confirmed the significance of the up-regulation of hemA, hemC/D, hemL, and of the LIMLP_17860-encoded histidine kinase of the TCS (Table 1 and S3 Fig).
When pathogenic Leptospira cells are exposed to 10 μM H2O2, the only ORF that was down-regulated was that encoding a permease (LIMLP_18600; with a Log2FC of -1). This permease is a putative Major Facilitator Superfamily (MFS) transporter and is predicted to contain 12 transmembrane helixes. This permease-encoding ORF is the second gene of a bicistronic operon where a heme oxygenase-encoding ORF (LIMLP_18595) is the first (S4 Fig). Expression of the heme oxygenase ORF was not significantly changed by the exposure to 10 μM H2O2 (S4 Fig and S1 Table).
Plotting statistical significance in function of fold change confirmed that katE, ccp, ahpC, perR, and several genes of the heme biosynthesis pathway were among the genes the expression of which was significantly up-regulated (Fig 1A).
Fig 1. Volcano representation of differentially-expressed genes upon exposure to hydrogen peroxide.
Up- and down-regulated genes upon a 30 min exposure to 10 μM H2O2 (A) or 1 hour exposure to 1 mM H2O2 (B) were graphically represented by a Volcano analysis. Red and blue dots indicate up- and down-regulated genes, respectively, with significant change in expression with a Log2FC cutoff of ±1 and p-value<0.05. Representative genes are labeled.
Notably, after a 2-hour exposure of L. interrogans to 10 μM H2O2, the expression of the peroxidases and heme biosynthesis genes returns to a level closer to that observed in the absence of H2O2 (S1–S4 Figs). Altogether, these data indicate that pathogenic Leptospira respond to a sublethal dose of H2O2 by soliciting three peroxidases and heme, and that the up-regulated peroxidase and catalase activities are probably sufficient to degrade H2O2 and allow survival of Leptospira in the conditions tested in this study.
Leptospira transcriptional response to lethal concentration of hydrogen peroxide
In order to better reproduce harmful oxidative stress encountered during infection, we performed similar RNA-Seq experiments upon 1-hour exposure to 1 mM H2O2. In this condition, Leptospira survival was 60% ± 2.735 as assessed by plating on EMJH agar plates. Comparison with untreated cells identified a total of 2145 genes with differential transcript abundance (see S2 Table for complete data set). Among those, 243 and 296 genes were respectively up- and down-regulated by ≥2.0 fold with p-values ≤0.05. The volcano representation exhibited more scattered data points (Fig 1B), bearing witness to a higher number of genes with significantly and statistically changed expression than when Leptospira are exposed to sublethal dose of H2O2 (Fig 1A).
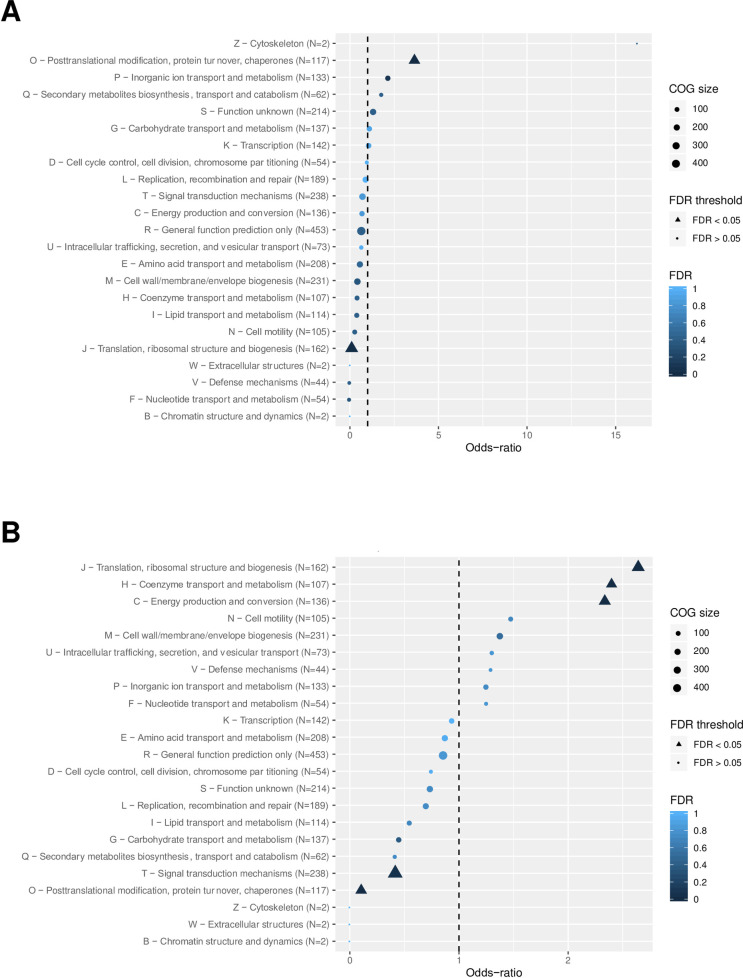
Differentially expressed genes were classified into COG functional categories and the obtained COG frequencies were compared to the frequency of the genes in the genome. As seen in Fig 2, the up-regulated genes were enriched in the post-translational modification, protein turnover, and chaperones categories whereas down-regulated genes mainly fell into metabolism, translation and ribosomal structure and biogenesis, coenzyme transport and metabolism, and energy production and conversion categories.
Fig 2. Classification of differentially-expressed genes upon exposure to lethal dose of hydrogen peroxide.
ORFs with significantly changed expression when L. interrogans were exposed to 1 mM H2O2 for 1h were classified according to the COG (Clusters of Orthologous Groups). A Log2FC cutoff of ±1 were applied for the up-regulated (A) and down-regulated (B) ORFs, respectively, with an adjusted p-value<0.005. An odd-ratio higher or lower than 1 (dashed line) indicates an over- or under-representation of a functional category, respectively, and a COG category with a False Discovery Rate (FDR) lower than 5% is considered as enriched. The functional categories are indicated on the left.
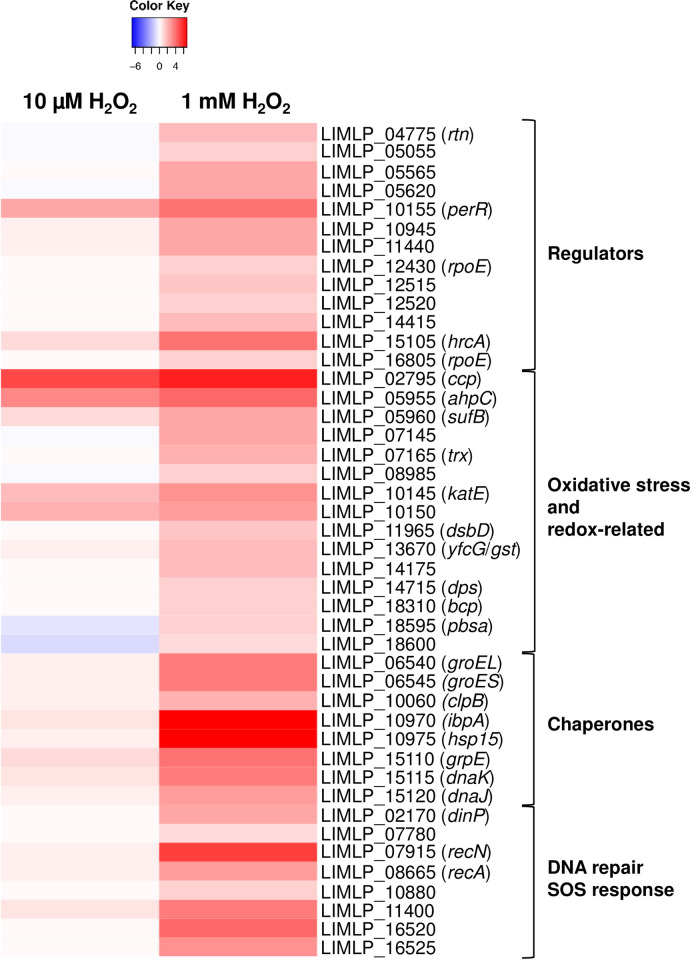
As in the presence of low dose of H2O2, the ank-katE operon (LIMLP_10150–10145), ccp (LIMLP_02795) and ahpC (LIMLP_05955) were up-regulated in the presence of 1 mM H2O2 but with higher fold changes (with Log2FC values of 2.7, 5.8 and 4, respectively, see Fig 3 and S3 Table). The ORF upstream ahpC that encodes a SufB (LIMLP_05960) was also significantly up-regulated (with a with Log2FC value of 2.2). Likewise, perR expression was greater in the presence of 1 mM H2O2 (with Log2FC value of 3.5, see Fig 3 and S3 Table). All these up-regulations were confirmed by RT-qPCR experiments (S3 Table).
Fig 3. Comparison of up-regulated genes upon exposure to sublethal and lethal doses of hydrogen peroxide.
The differential expression of selected up-regulated genes determined by RNA-Seq when L. interrogans are exposed 1 hour to 1 mM H2O2 was compared to that of L. interrogans exposed 30 min to 10 μM H2O2. Differential expression in H2O2-treated Leptospira was obtained by normalization with untreated Leptospira. Genes are organized by their function and their number and name are indicated on the right. The Heat Map color from blue to red indicates low to high Log2FC.
Additional ORFs encoding factors related to oxidative stress and redox maintenance were also up-regulated (Fig 3 and S2 and S3 Tables). An ORF encoding a thiol oxidoreductase (LIMLP_07145) exhibiting two cytochrome C-like (heme binding) domains was up-regulated with a Log2FC value of 2.2. LIMLP_07145 was located immediately downstream an ORF (LIMLP_07150) encoding a protein with five chromosome condensation regulator (RCC1) domains that was up-regulated with a Log2FC value of about 5. LIMLP_07145–07150 are probably organized as a bicistronic operon as predicted in Zhukova et al. [25]. A second thiol peroxidase-encoding ORF (LIMLP_14175) exhibiting a single cytochrome C-like domain was also up-regulated (Log2FC value of 1.8). This ORF might be part of the operon LIMLP_14170–14180 where LIMLP_14170 and LIMP_14180, two ORFs annotated as Imelysins (iron-regulated proteins), were also up-regulated (Log2FC value of 2.8 and 1.4, respectively). Of note, the Imelysin encoded by LIMLP_14170 is the LruB protein that was shown to be associated with Leptospira-induced uveitis [26].
A thioredoxin disulfide reductase (encoded by LIMLP_07165) was up-regulated (Log2FC value of 1.9, see Fig 3 and S3 Table). This protein has been shown to catalyze in vitro the NADPH-dependent reduction of a thioredoxin encoded by LIMLP_09870 [27]. The LIMLP_09870 was only slightly up-regulated in the presence of 1 mM H2O2 (Log2FC value of 0.8, see S2 Table).
Other thiol peroxidase-encoding ORFs were up-regulated, including LIMLP_08985 that encodes a glutaredoxin, LIMLP_11965 that codes for the periplasmic thiol disulfide interchange protein DsbD, and LIMLP_18310 that encodes a bacterioferritin comigratory protein (Bcp) (See Fig 3 and S3 Table). An ORF encoding a putative Glutathione S transferase (LIMLP_13670) had an increased expression in the presence of 1 mM H2O2 (Log2FC value of 1.76), as did an ORF annotated as DNA binding stress protein (Dps) (Log2FC value of 1.09) (See Fig 3 and S3 Table).
Major pathways involved in repair of damaged cellular components were dramatically up-regulated when Leptospira were exposed to a lethal dose of H2O2. Indeed, several genes encoding molecular chaperones had an increased expression in the presence of 1 mM H2O2 (Fig 3 and S3 Table). Two ORFs encoding small heat shock proteins (sHSP), probably organized as a bicistronic operon (LIMLP_10970–10975), exhibited a significant increase in expression (Log2FC values of about 6). The LIMLP_15105–15120 cluster encoding the DnaK/DnaJ/GrpE molecular chaperone machinery and its putative repressor HrcA, was significantly up-regulated with Log2FC values of 2.6–3.6. Similarly, the GroES-GroEL operon (encoded by LIMLP_06545–06540) was up-regulated with a Log2FC value of 3.3. The clpB gene (LIMLP_10060) also had an increased expression (Log2FC value of 2.1). Thus, the machinery necessary for preventing protein aggregation and promoting protein refolding is solicited when Leptospira are exposed to high doses of H2O2.
Genes encoding several components of the SOS response, a regulatory network stimulated by DNA damage-inducing stress, had a higher expression in the presence of 1 mM H2O2 (Fig 3 and S3 Table). Indeed, ORFs encoding the recombinase A (recA, LIMLP_08665), the DNA repair protein RecN (LIMLP_07915), the DNA polymerase IV (dinP, LIMLP_02170) as well as the repressor of the SOS response LexA1 (LIMLP_11440) were significantly up-regulated. Other factors putatively involved in DNA repair but not under the control of LexA1 [28,29] had also an increased expression, including the DNA mismatch repair protein MutS (LIMLP_07780, Log2FC value of 1) and the DNA repair protein RadC (LIMLP_11400, Log2FC value of 3.4).
One remarkably up-regulated ORF (LIMLP_00895) was located into a genomic region previously identified as an island enriched in prophage genes ranging from LIMLP_00855 to LIMLP_01005 and referred to as prophage 1 [29,30] (S3 Table). Also, another cluster enriched in prophage genes (from LIMLP_13010 to LIMLP_13095), referred to as prophage 2 [29], contains 4 ORFs (LIMLP_13010, LIMLP_13015, LIMLP_13020, and LIMLP_13025) that were up-regulated in the presence of 1 mM H2O2 (S3 Table).
Down-regulated genes were mainly genes putatively involved in translation and metabolism (Fig 2B and S4 Table). 14 ORFs encoding ribosomal proteins, a translation initiation factor (LIMLP_03190), a ribosome maturation factor (LIMLP_07600), a RNA polymerase RpoA (LIMLP_03215), and a transcription termination factor RhoA (LIMLP_13190) were among them.
A cluster of genes encoding the ATP synthase complex (LIMLP_06050–06080) was down-regulated, indicating that Leptospira decrease ATP synthesis upon exposure to high dose of H2O2 (S4 Table). Another metabolic pathway that was down-regulated in this condition was the cobalamin (vitamin B12) biosynthesis pathway. Indeed, 15 out 17 genes of the cobI/III cluster (LIMLP_18460–18530) were significantly down-regulated (S4 Table).
A cluster of four genes encoding proteins of the CRISPR-Cas machinery (csh2, LIMLP_2870; cas8, LIMLP_2875; cas5, LIMLP_2880; cas3, LIMLP_2880) putatively involved in phage defense were down-regulated (see S4 Table).
Finally, several genes related to motility/chemotaxis were down-regulated when Leptospira are exposed to a high dose of H2O2. Several of these genes encode constituents of the endoflagellum basal body (flgGAHIJ, LIMLP_06485–06505), of the flagellar export apparatus (fliOPQR-FlhBA, LIMLP_06690–06715; fliL, LIMLP_14615 and LIMLP_14620), and of the flagellar motor stator (motAB, LIMLP14625-14630), and chemotaxis-related proteins (cheBDW-mcp, LIMLP_07420–07435) (see S2 Table).
Contribution of PerR in the pathogenic Leptospira response to peroxide stress
Comparison of the transcriptome of a perR mutant with that of WT strain allowed determination of the PerR regulon in L. interrogans. In the perR mutant, 5 and 12 ORFs were up- and down-regulated, respectively, with a log2FC cutoff of 1 and a p-value below 0.05 (Table 2 and S1 Table).
Table 2. Differentially expressed genes upon perR inactivation.
| ORF IDa | Gene | Function | Log2FC | Adjusted p-value | FC (RT-qPCR)b |
|---|---|---|---|---|---|
| Down-regulated genes | |||||
| LIMLP_04090 (LIC12679/LA0980) | thic | Thiamine biosynthesis protein | -2.073 | 3.72e-02 | |
| LIMLP_04240 (LIC10889/LA3247) | tonb | Energy transducer TonB | -4.601 | 2.03e-13 | 0.00722 |
| LIMLP_04245 (LIC10890/LA3246) | exbD | Biopolymer transport protein ExbD/TolR | -4.606 | 7.90e-13 | 0.00737 |
| LIMLP_04250 (LIC10891/LA3245) | exbD | Biopolymer transport protein ExbD/TolR | -5.355 | 4.93e-15 | 0.00128 |
| LIMLP_04255 (LIC10892/LA3244) | exbB | Biopolymer transport protein ExbB/TolQ | -5.478 | 3.00e-22 | 0.00193 |
| LIMLP_04260 (LIC10893/LA3243) | Hypothetical | -1.519 | 4.27e-02 | 1.261 | |
| LIMLP_04270 (LIC10895-96/LA3242) | TonB-dependent receptor | -3.262 | 2.32e-05 | 0.0355 | |
| LIMLP_04275 (LIC10897/LA3241) | Hypothetical | -3.888 | 4.42e-05 | 0.00918 | |
| LIMLP_04280 (LIC10898/LA3240) | lipl48 | Hypothetical | -5.506 | 2.03e-13 | 0.00372 |
| LIMLP_09650 (LIC11935/LA1968)* | Hypothetical | -1.787 | 3.26e-02 | ||
| LIMLP_15470 (LIC10454/LA3793) | Putative hemolysin | -2.154 | 3.32e-12 | 0.3041 | |
| LIMLP_16720 (LIC13269/LA4102) | vicR | Response regulator | -1.611 | 5.80e-07 | 0.0752 |
| Up-regulated genes | |||||
| LIMLP_02010 (LIC13086/LA3867)** | Hypothetical lipoprotein | 1.029 | 4.08e-02 | ||
| LIMLP_02795 (LIC12927/LA0666)* | ccp | Cytochrome C peroxidase | 2.773 | 8.69e-18 | 7.943 |
| LIMLP_05955 (LIC11219/LA2809)* | ahpC | Peroxiredoxin/alkylperoxiredoxin reductase | 1.539 | 1.23e-05 | 2.01 |
| LIMLP_10145 (LIC12032/LA1859)* | katE | Catalase | 2.637 | 2.59e-24 | 4.897 |
| LIMLP_10150 (LIC12033/LA1858)* | Ankyrin repeat-containing protein | 2.867 | 4.65e-29 | 5.783 |
Significantly up-and down-regulated genes in the perR mutant with a Log2FC cutoff of ± 1 and an adjusted p-value cutoff of 0.05.
a Gene numeration is according to Satou et al. [17]. Corresponding genes of L. interrogans serovar lai strain 56601 and serovar Copenhageni strain Fiocruz L1-130 are indicated in parenthesis.
b Fold change in gene expression upon perR inactivation obtained by RT-qPCR experiments.
* ORFs significantly up-regulated upon exposure to H2O2 (Log2FC cutoff of -1, adjusted p-value cutoff of 0.05).
** ORFs significantly down-regulated upon exposure to H2O2 (Log2FC cutoff of -1, adjusted p-value cutoff of 0.05).
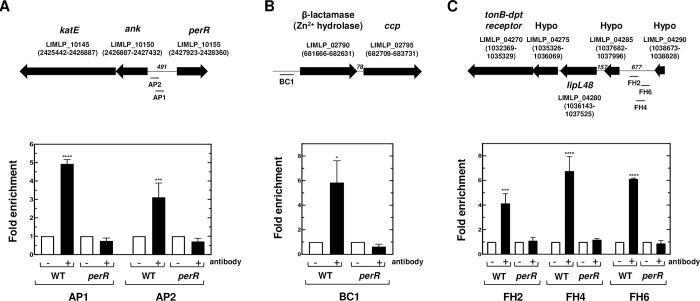
The ank-katE operon, encoded by LIMLP_10150–10145, ahpC, encoded by LIMLP_05955, and ccp, encoded by LIMLP_02795, were up-regulated upon perR inactivation. KatE, ahpC and ccp up-regulation is consistent with the high resistance of the perR mutant to H2O2 concentrations that are otherwise lethal for the WT strain [10,15]. ChIP-PCR experiments showed that when Leptospira were cultivated in EMJH medium, PerR was bound to DNA fragments comprising the 25 to 191 bp upstream region to the ank-katE operon (Fig 4A). Lower non-significant PerR binding was detected inside the ank-katE operon (S5 Fig). This is consistent with a direct repression of the ank-katE operon by PerR. Significant PerR binding was observed from 150 to 350 bp upstream the LIMLP_02790, the ORF located immediately upstream the ccp ORF (Fig 4B and S5 Fig). ChIP experiments also showed a rather weak binding upstream the ahpC ORF (-298 to -123 region) (S5 Fig).
Fig 4. In vivo interaction between PerR and promoter regions of PerR-controlled genes.
Chromatin immunoprecipitation was performed on L. interrogans WT and perR (M776) mutant strains in the presence or absence of the anti-PerR antibody as described in the Material and Methods section. Co-immunoprecipitated DNA fragments located in the ank-katE operon locus (A), in the ccp locus (B) and in the locus encoding a TonB-dependent transport system (C) were amplified by qPCR. The location of amplified fragments is indicated below the schematic representation of their respective locus. ORF location in the genome (according to Satou et al. [17]) is indicated into parenthesis and the number of nucleotides between different ORFs is indicated in italic. Data are represented as fold enrichments and are means and SD of two independent biological replicates (****, adjusted p-value<0.0001; ***, adjusted p-value of 0.0006; *, adjusted p-value of 0.0422).
Identification of the PerR regulon has allowed the identification of genes whose expression is activated directly or indirectly by PerR (Table 2). A cluster composed of genes encoding a TonB-dependent transport (TBDT) system (LIMLP_04240–04255, encoding TonB, two ExbDs and ExbB, respectively) was dramatically down-regulated in the perR mutant. The downstream TonB-dependent receptor- and LipL48-encoding ORFs were also down-regulated upon perR inactivation. LIMLP_04240 (tonB), LIMLP_04245 (exbD), and LIMLP_04250 (exbD) were organized as an operon and LIMLP_04270 (encoding the TonB-dependent receptor), LIMLP_04275, LIMLP_04280 (lipl48) and LIMLP_04285 constituted another operon (S6 Fig). ChIP-PCR assays indicated that a region upstream the LIMLP_04285–04270 operon (mapped from 436 to 617 bp) was significantly bound by PerR (Fig 4C) whereas lower binding was detected upstream the LIMLP_04265 ORF and within the LIMLP_04245 (S7 Fig). A bicistronic operon composed of the response regulator VicR (LIMLP_16720) and the histidine kinase VicK (LIMLP_16725) of a two-component system (S8 Fig) was also down-regulated (with a Log2FC of -1.61 and -0.91, respectively) (Table 2 and S1 Table).
Interestingly, among the PerR regulon, several genes whose expression is repressed by PerR were up-regulated when Leptospira were exposed to H2O2. Indeed, the expression of the ank-katE operon, ahpC and ccp were up-regulated in the perR mutant and in the presence of H2O2 whereas the expression of the ORFs encoding TonB, ExbD, ExbB, the TonB-dependent receptor, LipL48, VicK and VicR was not dramatically or significantly altered by the presence of H2O2.
In order to determine the exact contribution of PerR in the gene expression increase upon exposure to H2O2 in Leptospira, the transcriptome of the perR mutant exposed to a sublethal dose of H2O2 was also obtained (see S1 Table for a complete set of data). The ank-katE operon, whose expression is directly repressed by PerR and increased in the presence of H2O2 in WT Leptospira, was not up-regulated in the presence of H2O2 when perR was inactivated (Fig 5 and S1 Table). The amount of ank-katE operon expression in the perR mutant is in fact comparable to that in WT Leptospira exposed to a deadly dose of H2O2. This indicates that derepression of the ank-katE operon induced by the presence of H2O2 probably solely reflects PerR dissociation from DNA when PerR is oxidized. AhpC and ccp were still significantly up-regulated in the presence of H2O2 in the perR mutant (with Log2FC values of 2.30 and 1.88, respectively, see Fig 5 and S1 Table). Therefore, an H2O2-induced mechanism increases the expression of these two genes even in the absence of PerR, even though their expression is repressed by this regulator.
Fig 5. Comparison of differentially-expressed genes upon exposure to hydrogen peroxide and perR inactivation.
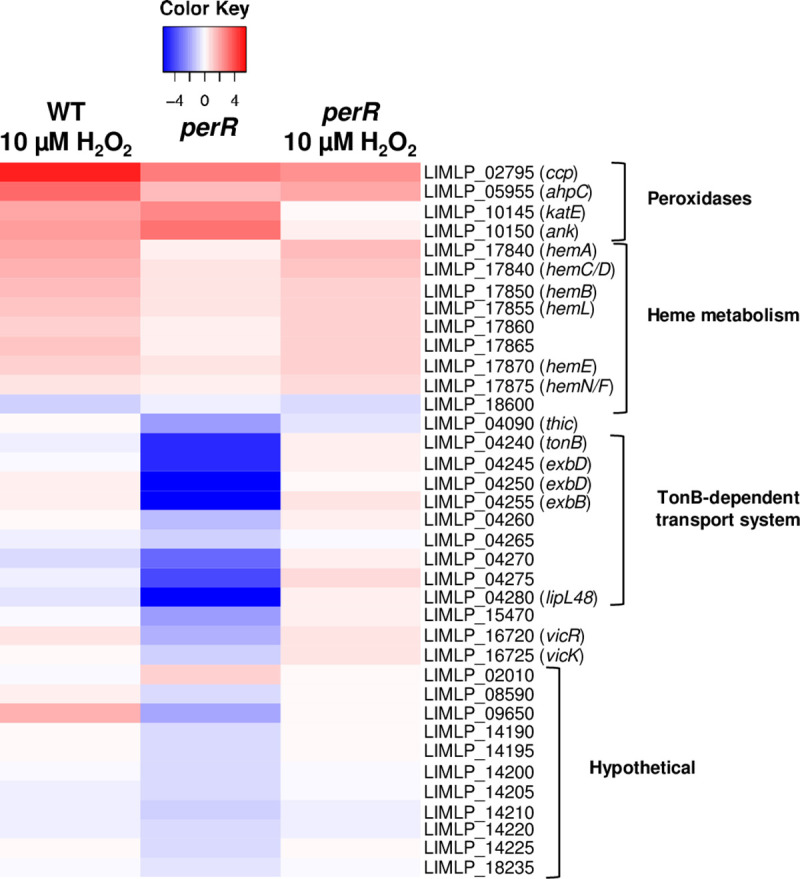
The expression of selected differentially-expressed genes determined by RNA-Seq when WT L. interrogans are exposed 30 min to 10 μM H2O2 was compared to that in the perR (M776) mutant in the absence or presence of 10 μM H2O2. Gene expression in the perR mutant strain with H2O2 was normalized here with that of the perR mutant strain without H2O2. Genes are organized by their function and their number and name are indicated on the right. The Heat Map color from blue to red indicates low to high Log2FC.
The expression of heme biosynthesis genes was not under the control of PerR and, as expected, their expression was still up-regulated in the perR mutant in the presence of H2O2 (Fig 5 and S1 Table).
Altogether, these findings indicate that not all H2O2-regulated genes belong to the PerR regulon in pathogenic Leptospira and several PerR-regulated genes were not regulated by H2O2 (Fig 5).
Identification of differentially expressed non-coding RNAs in the presence of hydrogen peroxide
In order to identify non-coding RNAs (ncRNAs) whose expression is changed in the presence of hydrogen peroxide, non-coding genome regions of RNA-Seq data were also analyzed. When Leptospira were exposed to 10 μM H2O2 for 30 min, only 19 ncRNAs were differentially expressed (see S5 Table and S6 Table for the complete set of data). The most highly up-regulated ncRNAs were rh859, rh3130 and rh3999 (S5 Table). When Leptospira were exposed to a lethal dose of hydrogen peroxide (1 mM H2O2 for 1h), a higher number of differentially expressed ncRNAs was detected. Indeed, 416 and 102 ncRNAs were up- and down-regulated, respectively (S6 Table). 63 ncRNAs were up-regulated with a Log2FC above 1. Rh3130 and rh3352 were the two most highly up-regulated ncRNAs with Log2FC above 7 and rh288 was up-regulated with a Log2FC of 3.81 (Table 3). 53 ncRNAs were down-regulated with a Log2FC below -1. Rh967 was among the most highly down-regulated ncRNAs with a Log2FC of -2.66.
Table 3. Differentially expressed ncRNAs upon exposure to lethal dose of H2O2.
| NC RNA | chromosome | Log2Fc | Adjusted p-value | Start-End | Overlapping ORFa | Upstream ORFa | Downstream ORFa | perR mutantb |
|---|---|---|---|---|---|---|---|---|
| Up-regulated | ||||||||
| rh34 | NZ_CP011933.1 | 1.218 | 1.23e-30 | 18632–19081 | LIMLP_19345 | LEPIMA _p0012 | LIMLP_19350 | |
| rh36 | NZ_CP011933.1 | 1.368 | 4.02e-37 | 20723–21129 | NA | LIMLP_19355 | LIMLP_19360 | |
| rh47 | NZ_CP011931.1 | 1.150 | 2.80e-57 | 37221–37455 | NA | LIMLP_00160 | LIMLP_00165 | |
| rh49 | NZ_CP011932.1 | 1.013 | 2.50e-47 | 39710–39825 | LEPIMA_CII0041 | LIMLP_18000 | LIMLP_18005 | |
| rh57§ | NZ_CP011933.1 | 1.629 | 1.95e-69 | 23941–24050 | LEPIMA _p0025 | LIMLP_19380 | LIMLP_19385 | |
| rh82 | NZ_CP011933.1 | 1.362 | 3.50e-37 | 32192–32351 | NA | LIMLP_19435 | LEPIMA _p0038 | |
| rh97 | NZ_CP011932.1 | 1.170 | 3.03e-57 | 66008–66133 | LIMLP_18130 | LIMLP_18125 | LEPIMA_CII0073 | |
| rh178 | NZ_CP011933.1 | 1.195 | 2.92e-22 | 57959–58185 | NA | LEPIMA _p0081 | LIMLP_19600 | |
| rh179 | NZ_CP011933.1 | 1.082 | 7.04e-14 | 58957–59117 | LEPIMA _p0083 | LIMLP_19600 | LIMLP_19605* | |
| rh183 | NZ_CP011931.1 | 1.557 | 7.61e-80 | 128941–129006 | NA | LIMLP_00580 | LIMLP_00585 | |
| rh184 | NZ_CP011931.1 | 1.228 | 2.09e-66 | 129119–129450 | LIMLP_00585 | LIMLP_00580 | LIMLP_00595 | |
| rh199 | NZ_CP011933.1 | 1.009 | 7.80e-24 | 63659–64935 | LEPIMA_p0089, LIMLP_19625, LIMLP_19630 | LIMLP_19620 | LIMLP_19635 | |
| rh210 | NZ_CP011931.1 | 1.231 | 1.22e-61 | 158886–159090 | NA | LIMLP_00700* | LIMLP_00705 | |
| rh219 | NZ_CP011932.1 | 1.337 | 2.22e-97 | 147192–147336 | LIMLP_18455** | LIMLP_18450 | LIMLP_18460** | |
| rh288 | NZ_CP011931.1 | 3.812 | 0.00 | 197282–197352 | LIMLP_00895* | LIMLP_00890 | LEPIMA_CI0185 | Up-regulated |
| rh349 | NZ_CP011932.1 | 1.638 | 1.98e-120 | 256577–256639 | NA | LEPIMA_CII0243 | LIMLP_18855 | |
| rh402 | NZ_CP011932.1 | 1.517 | 1.31e-92 | 291497–291605 | LIMLP_18995 | LIMLP_18990** | LIMLP_19000* | |
| rh449 | NZ_CP011931.1 | 2.512 | 0.00 | 351592–351649 | NA | LIMLP_01545* | LIMLP_01550* | |
| rh488 | NZ_CP011932.1 | 1.184 | 1.11e-48 | 352594–352673 | LIMLP_19275 | LIMLP_19270 | LIMLP_19280 | |
| rh490 | NZ_CP011932.1 | 1.277 | 2.18e-49 | 353695–353790 | LIMLP_19280 | LIMLP_19275 | LIMLP_19285 | |
| rh593 | NZ_CP011931.1 | 1.362 | 1.44e-43 | 471763–471828 | LIMLP_02010** | LIMLP_02005 | LEPIMA_CI0422 | |
| rh608 | NZ_CP011931.1 | 2.476 | 0.00 | 479394–479448 | LIMLP_02045* | LIMLP_02040* | LIMLP_02050 | |
| rh625 | NZ_CP011931.1 | 1.077 | 9.15e-49 | 499440–499814 | LIMLP_02100 | LIMLP_02095 | LIMLP_02105* | |
| rh637# | NZ_CP011931.1 | 1.663 | 2.16e-137 | 501388–501477 | NA | LIMLP_02105* | LIMLP_02110 | |
| rh786 | NZ_CP011931.1 | 1.135 | 6.17e-50 | 632024–632186 | LIMLP_02580 | LIMLP_02575 | LIMLP_02585 | |
| rh859# | NZ_CP011931.1 | 4.248 | 0.00 | 683752–684074 | NA | LIMLP_02795* | LEPIMA_CI0612 | Up-regulated |
| rh1048 | NZ_CP011931.1 | 1.059 | 3.63e-43 | 846807–846960 | LIMLP_03520 | LIMLP_03515 | LIMLP_03525 | |
| rh1167 | NZ_CP011931.1 | 1.070 | 2.97e-37 | 943926–943989 | LIMLP_03935 | LIMLP_03930 | LIMLP_03940 | |
| rh1192 | NZ_CP011931.1 | 2.197 | 5.78e-214 | 975150–975213 | LIMLP_04030 | LIMLP_04025 | LIMLP_04035 | |
| rh1210 (RF00059) | NZ_CP011931.1 | 1.236 | 3.67e-37 | 995004–995065 | NA | LIMLP_04090 | LIMLP_04095** | |
| rh1269 | NZ_CP011931.1 | 2.164 | 2.97e-202 | 1038822–1038876 | LIMLP_04290 | LIMLP_04285 | LIMLP_04295* | |
| rh1270 | NZ_CP011931.1 | 1.491 | 2.13e-108 | 1039034–1039628 | LIMLP_04295*, LEPIMA_CI0938 | LIMLP_04290 | LIMLP_04300 | |
| rh1429 | NZ_CP011931.1 | 1.720 | 3.15e-90 | 1181397–1181456 | LIMLP_04840 | LIMLP_04830 | LIMLP_04845 | |
| rh1498 | NZ_CP011931.1 | 1.288 | 1.08e-46 | 1260173–1260234 | NA | LEPIMA_CI1128 | LIMLP_05125* | |
| rh1641 | NZ_CP011931.1 | 2.013 | 2.50e-145 | 1386755–1386830 | LIMLP_05625 | LIMLP_05620* | LIMLP_05630 | |
| rh1807 | NZ_CP011931.1 | 2.928 | 0.00 | 1531048–1531289 | LIMLP_06235 | LIMLP_06230 | LIMLP_06240 | |
| rh2088 | NZ_CP011931.1 | 2.000 | 1.66e-149 | 1780300–1780403 | LIMLP_07195 | LEPIMA_CI1612 | LIMLP_07200 | |
| rh2227 | NZ_CP011931.1 | 3.130 | 0.00 | 1892070–1892135 | NA | LIMLP_07695 | LIMLP_07700 | |
| rh2395 | NZ_CP011931.1 | 1.877 | 2.09e-123 | 2013277–2013341 | LIMLP_08295 | LIMLP_08290 | LIMLP_08300 | |
| rh2487 | NZ_CP011931.1 | 1.230 | 5.68e-66 | 2083779–2083898 | LIMLP_08585 | LEPIMA_CI1903 | LIMLP_08590* | Down-regulated |
| rh2961 | NZ_CP011931.1 | 1.974 | 8.15e-150 | 2474618–2474668 | LIMLP_10350 | LIMLP_10345 | LIMLP_10355 | |
| rh3130# | NZ_CP011931.1 | 7.189 | 0.00 | 2612368–2612495 | LEPIMA_CI2416 | LIMLP_10975* | LEPIMA_CI2417 | |
| rh3147 | NZ_CP011931.1 | 1.230 | 8.22e-39 | 2625684–2625735 | LIMLP_11030 | LIMLP_11025 | LEPIMA_CI2429 | |
| rh3352# | NZ_CP011931.1 | 7.653 | 0.00 | 2787780–2787953 | LIMLP_11710* | LIMLP_11705 | LIMLP_11715* | |
| rh3535 | NZ_CP011931.1 | 1.016 | 1.77e-49 | 2958335–2958610 | NA | LIMLP_12420 | LIMLP_12425* | |
| rh3538 | NZ_CP011931.1 | 1.000 | 2.78e-21 | 2958938–2959002 | LIMLP_12425* | LIMLP_12420 | LIMLP_12430* | |
| rh3726 | NZ_CP011931.1 | 1.010 | 5.62e-46 | 3123653–3123980 | LIMLP_13150 | LIMLP_13145* | LIMLP_13155 | |
| rh3831 | NZ_CP011931.1 | 1.060 | 1.74e-26 | 3214862–3214919 | LIMLP_13525 | LIMLP_13520 | LIMLP_13530 | |
| rh3871 | NZ_CP011931.1 | 2.133 | 2.34e-261 | 3253035–3253139 | LIMLP_13675 | LIMLP_13670* | LIMLP_13680 | |
| rh3894 | NZ_CP011931.1 | 3.784 | 0.00 | 3271638–3271704 | NA | LIMLP_13765* | LIMLP_13770 | |
| rh4111 | NZ_CP011931.1 | 1.340 | 2.82e-76 | 3446561–3446742 | LIMLP_14535 | LIMLP_14530 | LEPIMA_CI3186 | |
| rh4124 | NZ_CP011931.1 | 1.233 | 3.41e-66 | 3459090–3459232 | NA | LIMLP_14580 | LIMLP_14585* | |
| rh4168 | NZ_CP011931.1 | 1.244 | 4.53e-58 | 3487314–3487435 | NA | LIMLP_14710 | LIMLP_14715* | |
| rh4281 | NZ_CP011931.1 | 1.627 | 1.38e-71 | 3584015–3584072 | LIMLP_15080** | LIMLP_15075** | LIMLP_15085 | |
| rh4345 | NZ_CP011931.1 | 1.765 | 8.56e-126 | 3664279–3664343 | LIMLP_15310 | LIMLP_15305 | LIMLP_15315** | |
| rh4413# | NZ_CP011931.1 | 3.507 | 0.00 | 3721204–3721564 | NA | LIMLP_15540* | LIMLP_15545 | |
| rh4459# | NZ_CP011931.1 | 1.059 | 6.95e-29 | 3755947–3756010 | NA | LIMLP_15710 | LEPIMA_CI3455 | |
| rh4542 | NZ_CP011931.1 | 2.748 | 0.00 | 3822746–3823025 | LIMLP_16010 | LIMLP_16005 | LIMLP_16015* | |
| rh4545 | NZ_CP011931.1 | 1.979 | 1.54e-233 | 3825144–3825319 | NA | LIMLP_16015* | LIMLP_16025* | |
| rh4746 | NZ_CP011931.1 | 1.222 | 1.77e-36 | 3987147–3987296 | LEPIMA_CI3684 | LIMLP_16760 | LIMLP_16765* | |
| rh4747 | NZ_CP011931.1 | 1.178 | 9.32e-60 | 3987366–3987576 | LEPIMA_CI3684 | LIMLP_16760 | LIMLP_16765* | |
| rh4854 (RF00174) | NZ_CP011931.1 | 1.023 | 9.20e-41 | 4078407–4078514 | NA | LIMLP_17135 | LIMLP_17140 | |
| rh5034 | NZ_CP011931.1 | 1.628 | 2.24e-72 | 4229144–4229208 | NA | LIMLP_17780 | LIMLP_17785 | |
| Down-regulated | ||||||||
| rh38 | NZ_CP011932.1 | -1.078 | 2.65e-08 | 32083–32148 | LIMLP_17965 | LIMLP_17960 | LIMLP_17970 | |
| rh81 | NZ_CP011931.1 | -1.246 | 3.21e-11 | 67349–67433 | NA | LIMLP_00285 | LIMLP_00290 | |
| rh278 | NZ_CP011932.1 | -1.188 | 8.73e-13 | 202039–202120 | NA | LIMLP_18675 | LEPIMA_CII0202 | |
| rh331 | NZ_CP011931.1 | -1.137 | 9.15e-08 | 245678–245742 | NA | LIMLP_01140 | LEPIMA_CI0236 | |
| rh411 | NZ_CP011931.1 | -1.854 | 1.53e-65 | 310470–310529 | NA | LIMLP_01410** | LIMLP_01415** | |
| rh418 | NZ_CP011931.1 | -1.395 | 9.73e-15 | 317250–317316 | NA | LIMLP_01445 | LIMLP_01450 | |
| rh429 | NZ_CP011932.1 | -1.375 | 2.93e-11 | 311436–311731 | NA | LIMLP_19090 | LIMLP_19095 | |
| rh589 | NZ_CP011931.1 | -1.000 | 3.55e-07 | 469227–469400 | NA | LIMLP_01995 | LIMLP_02000 | |
| rh685 | NZ_CP011931.1 | -1.613 | 1.73e-40 | 541558–541624 | NA | LEPIMA_CI0489 | LIMLP_02275 | |
| rh697 | NZ_CP011931.1 | -1.276 | 3.61e-11 | 549563–549676 | NA | LIMLP_02295 | LIMLP_02300 | |
| rh698 | NZ_CP011931.1 | -1.170 | 8.45e-09 | 549730–549828 | NA | LIMLP_02295 | LIMLP_02300 | |
| rh711 | NZ_CP011931.1 | -1.080 | 1.30e-07 | 562570–562701 | NA | LIMLP_02335 | LIMLP_02340 | |
| rh736 | NZ_CP011931.1 | -1.474 | 3.95e-17 | 582458–582528 | NA | LIMLP_02395** | LIMLP_02400** | |
| rh753 | NZ_CP011931.1 | -1.226 | 1.94e-13 | 602773–602842 | NA | LIMLP_02460 | LIMLP_02465 | |
| rh784 | NZ_CP011931.1 | -1.103 | 1.10e-10 | 630185–630381 | LIMLP_02570 | LIMLP_02565 | LIMLP_02575 | |
| rh967 | NZ_CP011931.1 | -2.662 | 8.54e-202 | 786700–786893 | NA | LIMLP_03220** | LIMLP_03225 | |
| rh1008 | NZ_CP011931.1 | -1.145 | 5.44e-10 | 819424–819662 | NA | LIMLP_03375 | LIMLP_03380 | |
| rh1101 | NZ_CP011931.1 | -2.684 | 4.82e-295 | 888430–888480 | NA | LIMLP_03700 | LIMLP_03705** | |
| rh1102 | NZ_CP011931.1 | -2.149 | 2.11e-80 | 888546–888608 | NA | LIMLP_03700 | LIMLP_03705** | |
| rh1140 | NZ_CP011931.1 | -1.188 | 5.61e-08 | 920214–921272 | NA | LIMLP_03840 | LIMLP_03845 | |
| rh1142 | NZ_CP011931.1 | -1.157 | 6.58e-10 | 920631–920965 | NA | LIMLP_03840 | LIMLP_03845 | |
| rh1253 | NZ_CP011931.1 | -1.608 | 1.10e-30 | 1025093–1025156 | LEPIMA_CI0924 | LEPIMA_CI0923 | LEPIMA_CI0925 | |
| rh1282 | NZ_CP011931.1 | -1.367 | 4.09e-15 | 1046519–1046574 | LEPIMA_CI0946 | LIMLP_04325** | LIMLP_04330 | |
| rh1299 | NZ_CP011931.1 | -1.054 | 9.24e-08 | 1057522–1057700 | LEPIMA_CI0958 | LIMLP_04385 | LIMLP_04390 | |
| rh1382 | NZ_CP011931.1 | -1.036 | 1.69e-05 | 1129722–1129784 | NA | LEPIMA_CI1022 | LEPIMA_CI1023 | |
| rh1651 | NZ_CP011931.1 | -1.354 | 4.48e-12 | 1392744–1392884 | LIMLP_05660 | LIMLP_05655 | LIMLP_05665 | |
| rh1880 | NZ_CP011931.1 | -1.896 | 6.86e-62 | 1592557–1592621 | LEPIMA_CI1441 | LIMLP_06480** | LEPIMA_CI1442 | |
| rh2038 | NZ_CP011931.1 | -1.252 | 1.81e-14 | 1734004–1734144 | NA | LIMLP_07030 | LIMLP_07035 | |
| rh2114 | NZ_CP011931.1 | -1.206 | 2.39e-12 | 1799567–1799634 | NA | LIMLP_07290 | LIMLP_07295 | |
| rh2170 | NZ_CP011931.1 | -1.047 | 1.58e-06 | 1854827–1854890 | LEPIMA_CI1676 | LIMLP_07495 | LIMLP_07500 | |
| rh2222 | NZ_CP011931.1 | -1.199 | 1.09e-12 | 1896532–1896782 | NA | LIMLP_07715 | LIMLP_07725 | |
| rh2311 | NZ_CP011931.1 | -1.115 | 1.01e-06 | 1954525–1954640 | LIMLP_07975 | LIMLP_07970** | LIMLP_07980 | |
| rh2578 | NZ_CP011931.1 | -1.730 | 2.54e-44 | 2165614–2165832 | LIMLP_08925 | LIMLP_08920 | LIMLP_08930** | |
| rh2850 | NZ_CP011931.1 | -1.378 | 1.21e-14 | 2378010–2378075 | LEPIMA_CI2192 | LIMLP_09945 | LIMLP_09950 | |
| rh2882 | NZ_CP011931.1 | -1.299 | 2.18e-12 | 2398625–2398696 | NA | LIMLP_10050** | LEPIMA_CI2213 | |
| rh3186 | NZ_CP011931.1 | -1.963 | 5.74e-64 | 2658407–2658646 | NA | LIMLP_11175** | LIMLP_11180** | |
| rh3190 | NZ_CP011931.1 | -1.874 | 6.42e-47 | 2656130–2656312 | NA | LIMLP_11170** | LIMLP_11175** | |
| rh3335 | NZ_CP011931.1 | -1.382 | 4.99e-21 | 2764692–2764792 | NA | LIMLP_11630 | LIMLP_11635 | |
| rh3711 | NZ_CP011931.1 | -2.030 | 5.35e-87 | 3116206–3116269 | NA | LIMLP_13120 | LEPIMA_CI2881 | |
| rh3945 | NZ_CP011931.1 | -1.465 | 7.13e-22 | 3308829–3309056 | LEPIMA_CI3062 | LIMLP_13935 | LIMLP_13940 | |
| rh3946 | NZ_CP011931.1 | -1.126 | 3.52e-10 | 3309111–3309181 | NA | LEPIMA_CI3062 | LIMLP_13940 | |
| rh4140 | NZ_CP011931.1 | -1.438 | 7.92e-17 | 3467363–3467432 | NA | LIMLP_14615** | LIMLP_14620** | |
| rh4178 | NZ_CP011931.1 | -1.509 | 2.89e-18 | 3496010–3496183 | LEPIMA_CI3239 | LIMLP_14745** | LIMLP_14750 | |
| rh4218 | NZ_CP011931.1 | -1.174 | 6.15e-08 | 3532306–3532358 | NA | LEPIMA_CI3268 | LIMLP_14880 | |
| rh4253 | NZ_CP011931.1 | -1.332 | 9.51e-14 | 3554072–3554567 | NA | LIMLP_14970** | LIMLP_14975 | |
| rh4254 | NZ_CP011931.1 | -1.246 | 1.77e-09 | 3554619–3554683 | NA | LIMLP_14970** | LIMLP_14975 | |
| rh4493 | NZ_CP011931.1 | -1.115 | 2.32e-09 | 3783194–3783362 | NA | LIMLP_15840 | LIMLP_15845 | |
| rh4549 | NZ_CP011931.1 | -1.821 | 7.63e-51 | 3827129–3827377 | LEPIMA_CI3525 | LIMLP_16030 | LIMLP_16035** | |
| rh4607 | NZ_CP011931.1 | -1.280 | 4.56e-14 | 3879350–3879511 | LIMLP_16285 | LIMLP_16280 | LIMLP_16290 | |
| rh4763 | NZ_CP011931.1 | -1.116 | 3.50e-03 | 3984140–3984338 | LIMLP_16745** | LIMLP_16740 | LIMLP_16750 | |
| rh4894 | NZ_CP011931.1 | -1.137 | 3.70e-11 | 4116602–4116674 | LIMLP_17290 | LIMLP_17285 | LIMLP_17295 | |
| rh4918 | NZ_CP011931.1 | -1.256 | 5.77e-17 | 4133590–4133654 | NA | LIMLP_17350 | LIMLP_17355 | |
| rh4938 | NZ_CP011931.1 | -1.064 | 3.10e-08 | 4155394–4155481 | LIMLP_17425** | LIMLP_17420** | LIMLP_17430** |
Significantly differentially-expressed ncRNAs upon 1h exposure to 1 mM H2O2 with Log2FC cutoff of ± 1.0 and a p-value cutoff of 0.05.
a Gene numeration is according to to Satou et al. [17].
b Differential expression of ncRNA upon inactivation of perR (M776 mutant) (see S5 Table).
# ncRNAs significantly up-regulated upon a 30 min exposure to 10 μM H2O2 (Log2FC cutoff of 1, p-value cutoff of 0.05, see S5 Table).
§ ncRNA significantly down-regulated upon a 30 min exposure to 10 μM H2O2 (Log2FC cutoff of -1, p-value cutoff of 0.05, see S5 Table).
* ORFs significantly up-regulated by RNASeq analysis (Log2FC cutoff of 1, adjusted p-value cutoff of 0.05).
** ORFs significantly down-regulated by RNASeq analysis (Log2FC cutoff of -1, adjusted p-value cutoff of 0.05).
NA, non-applicable
The Rfam classification of ncRNAs is indicated into parenthesis.
Several of the ncRNAS whose expression was up- or down-regulated in the presence of hydrogen peroxide were located in the vicinity or overlapped ORFs that were also up- or down-regulated in the same conditions. For instance, the rh3130 and rh859, among the most highly up-regulated ncRNAs, were in the vicinity of Hsp20 and CCP-encoding ORFs (LIMLP_10970–10975 and LIMLP_02795, respectively), three genes whose expression was greatly increased in the presence of hydrogen peroxide (Tables 1 and 3 and Fig 3). LIMLP_05620, LIMLP_13670, and LIMLP_13765 were three up-regulated ORFs upon exposure to hydrogen peroxide that have a downstream ncRNA (rh1641, rh3871, and rh3894, respectively). The up-regulated rh288 overlapped with the H2O2-induced LIMLP_00895, an ORF located in the prophage locus 1 (Table 3). This tendency was also observed with down-regulated ncRNAs. Rh411, rh967, rh1101, rh1102, rh1880, rh3186, and rh4281 ncRNAs were also located downstream or upstream, or overlapped ORFs whose expression was decreased in the presence of hydrogen peroxide (Table 3).
Three ncRNAs were noticeably differentially expressed upon perR inactivation. Rh288 and rh859 were up-regulated and rh1263 (located in the intergenic region upstream the TonB/ExbD2/ExbB-encoding operon, LIMLP_04255–04240) was significantly down-regulated in the perR mutant (S5 Table). Interestingly, the ncRNA rh859 was still up-regulated in the perR mutant upon exposure to H2O2 (S5 Table). This indicates that the rh859 up-regulation induced by the exposure of WT Leptospira to H2O2 occurs to some extent independently of the presence of PerR.
Most of the predicted ncRNAs show little homology with well-characterized RNAs families of the RFam database (S6 Table). However, this study has allowed the identification of a putative TPP riboswitch (rh1210; RFam 00059), three putative cobalamin riboswitches (rh1913, rh3382, rh4854; RFam 00174), a putative AsrC (Antisense RNA of rseC) (rh2876; RFam 02746) and a putative ligA thermometer (rh1488; RFam02815). Only the putative TPP (rh1210) and cobalamin (rh4854) riboswitches were up-regulated upon Leptospira exposure to H2O2. Further experiments will be needed to confirm the existence of these putative ncRNAs and establish their function in Leptospira physiology and virulence. Genetic manipulation of pathogenic Leptospira is still a challenge and functional studies in these bacteria mainly relies on random transposon insertion. Our laboratory has constructed a transposon mutant library [31], however no mutant in the putative ncRNAs is yet available in our random transposon mutant library.
Altogether, these findings indicate that exposure of Leptospira to 1 mM H2O2 triggers a drastic change in the expression of putative ncRNAs that correlates with dramatic changes in coding sequence expression.
Role of the PerR-regulated genes in defenses against ROS and virulence in Leptospira
RNA-Seq experiments have allowed the identification of differentially expressed ORFs in the presence of peroxide and upon perR inactivation. These ORFs might encode factors required for the adaptation of pathogenic Leptospira to ROS and an important question is to experimentally establish and understand the role of these factors in this adaptation. Several mutants inactivated in differentially-expressed ORFs upon exposure to H2O2 or upon perR inactivation were available in our transposon mutant library.
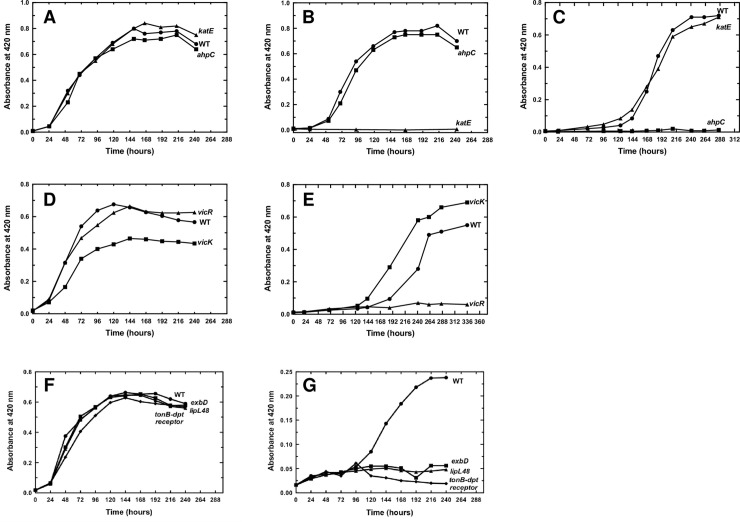
Catalase, AhpC, and CCP were the peroxidases up-regulated in the presence of H2O2 and repressed by PerR. Only katE and ahpC mutants were available in the transposon mutant library and we have studied the ability of these mutants to grow in the presence of H2O2 and paraquat, a superoxide-generating compound. These two mutants had a comparable growth rate in EMJH medium (Fig 6A) but when the medium was complemented with 0.5 mM H2O2, the ability of the katE mutant to divide was dramatically impaired (Fig 6B). The growth rate of the ahpC mutant in the presence of H2O2 was comparable to that of the WT strain (Fig 6B). When the EMJH medium was complemented with 2 μM paraquat, the growth of the ahpC mutant was considerably reduced, indicating a high sensitivity to superoxide (Fig 6C).
Fig 6. Effect of the inactivation of PerR-controlled genes on Leptospira growth in the presence of ROS.
L. interrogans WT, katE (Man69) and ahpC (Man1368), vicK (Man1448) and vicR (Man899), tonB-dpt receptor (Man1022), exbD (Man782), and lipl48 (Man1089) mutant strains were cultivated in EMJH medium (A, D, F) or in the presence of 2 mM H2O2 (B) or of 2 μM paraquat (C, E, G). Growth was assessed by measure of absorbance at 420 nm.
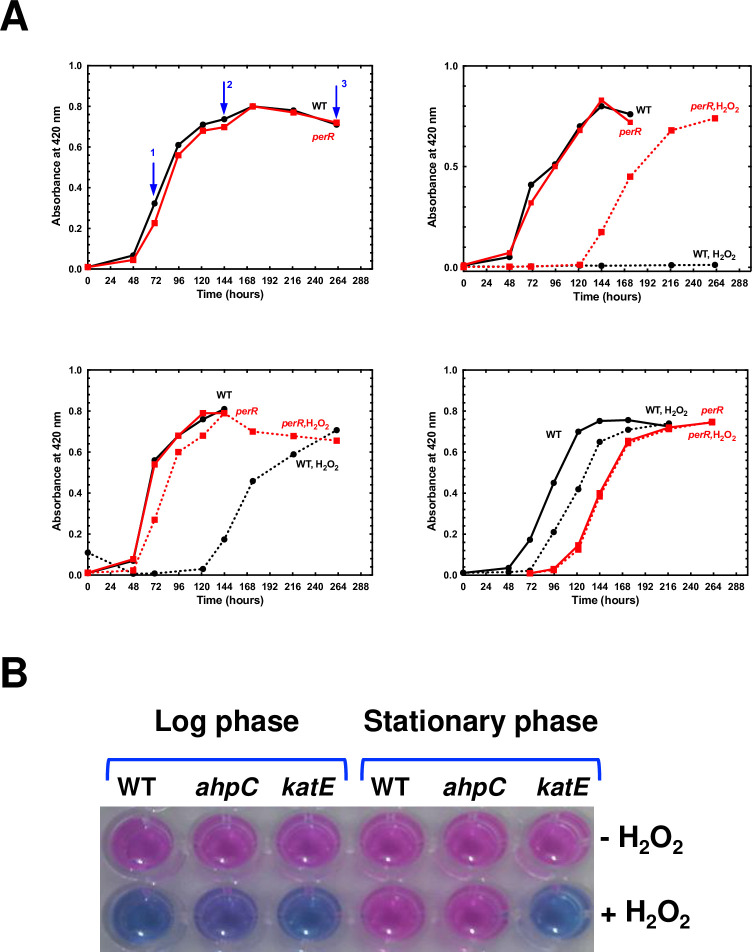
In other bacteria including E. coli and B. subtilis, katE is produced in higher amount during stationary phase [32,33], and in order to further characterize the role of katE in Leptospira survival under oxidative stress, we investigated the survival of stationary phase-adapted Leptospira in the presence of H2O2. L. interrogans WT cells were cultivated in EMJH medium and samples were harvested in the logarithmic phase (Fig 7A, sample 1), at the entry in stationary phase (Fig 7A, sample 2) and in late stationary phase (Fig 7A, sample 3). Each sample was used to inoculate a new batch of EMJH medium in the absence or presence of 2 mM H2O2. As seen in Fig 7A, when EMJH was inoculated with Leptospira WT strain at logarithmic phase, Leptospira were not able to divide in the presence of 2 mM H2O2. However, when the culture medium was inoculated with Leptospira WT strain at the beginning of the stationary phase, Leptospira acquired a greater resistance to 2 mM H2O2 as seen by their ability to grow (Fig 7A). An even higher ability to grow in the presence of a deadly dose of H2O2 was observed when the EMJH medium was inoculated with Leptospira at late stationary phase (Fig 7A). This indicates that Leptospira acquire a higher tolerance to hydrogen peroxide at stationary phase. Interestingly, this acquired tolerance to H2O2 was independent of PerR since the perR mutant also acquired a higher ability to grow in the presence of 2 mM H2O2 when at stationary phase (Fig 7A). In order to determine which peroxidase was responsible for this acquired tolerance to H2O2, the survival of WT, ahpC and katE mutant strains was tested in logarithmic phase and was compared with that in stationary phase. As seen in Fig 7B, a 30 min exposure to 10 mM H2O2 led to dramatic loss of survival of all strains at logarithmic phase. WT and ahpC mutant strains were able to acquire a higher resistance to H2O2 when placed at stationary phase whereas the katE mutant did not. Therefore, katE is essential for the stationary phase-acquired resistance to H2O2 and this probably involves another regulation mechanism than that exerted by PerR.
Fig 7. Role of catalase and AhpC in the stationary phase-adapted Leptospira tolerance to hydrogen peroxide.
(A) L. interrogans WT (black line) and perR mutant (M776) (red line) strains were cultivated in EMJH medium and samples were taken at the exponential phase (at OD420 nm ≈ 0.3, left upper panel, blue arrow 1), at the entry of stationary phase (at OD420 nm ≈ 0.7, left upper panel, blue arrow 2), and at late stationary phase (at OD420 nm ≈ 0.7, 5 days after the entry in stationary phase, left upper panel, blue arrow 3) and used to inoculate a new EMJH medium in the absence (plain line) or presence of 2 mM H2O2 (dashed line). The growth curve with samples taken in the exponential phase (samples 1), in the entry of stationary phase (samples 2) and at late stationary phase (samples 3) are represented in the right upper, the left lower, and the right lower panels, respectively. (B) L. interrogans WT, katE (Man69) and ahpC (Man1368) mutant strains were cultivated in EMJH medium until the exponential or stationary phases and incubated for 30 min in the absence or presence of 10 mM H2O2. Cell viability was assessed by the ability of the cells to reduce the blue rezasurin into a pink resorufin using the Alamar Blue assay as described in the Material and Methods section.
Among the genes repressed by PerR, only mutants inactivated in LIMLP_04245 (exbD), LIMLP_04270 (tonB-dpt receptor), LIMLP_04280 (lipl48), LIMLP_16720 (vicR), and LIMLP_16725 (vicK), were available in the transposon mutant library. All these mutants but vicK had a growth rate comparable to that of the WT strain in EMJH medium (Fig 6D and 6F). Despite the fact that vicK had a reduced ability to divide in EMJH medium, this mutant strain had a slightly greater resistance to 2 μM paraquat than that of the WT (Fig 6E). In the same condition, the vicR, exbD, tonB-dpt receptor, and lipl48 mutant strains had a lower ability to grow than the WT strain (Fig 6E and 6G). Altogether, these findings suggest that some of the PerR-repressed ORFs are involved in Leptospira defense against superoxide.
Catalase has been shown to be essential for Leptospira virulence [9]. We investigated whether other PerR-controlled genes were also required for Leptospira virulence. The different mutants were used in infection experiments in the acute model for leptospirosis. VicK, exbD, and lipl48 mutants did not exhibit dramatically altered virulence when 106 bacteria were injected peritoneally in hamsters (Fig 8A and 8B). In order to further challenge the role of the TonB-dependent transport system in Leptospira virulence, we tested whether a lower dose of infection with the tonB-dpt receptor and exbD mutants would result in a virulence attenuation. As seen in Fig 8C, when 104 bacteria were injected peritoneally in hamsters, animals infected with the exbD mutant exhibited 25% survival at 32 days post infection with no sign of leptospirosis. However, this slight virulence attenuation is not statistically significant. Therefore, inactivation of the TonB-dependent transport system or of the two-component system VicKR does not have a drastic consequence on Leptospira virulence in the acute model of infection in the conditions used in this study. These mechanisms do not have a pivotal role in Leptospira during infection or redundant activities compensate for their absence. Experiments using other infection routes (ocular or subcutaneous routes) might result in different outcomes.
Fig 8. Role of PerR-controlled ORFs in Leptospira virulence.
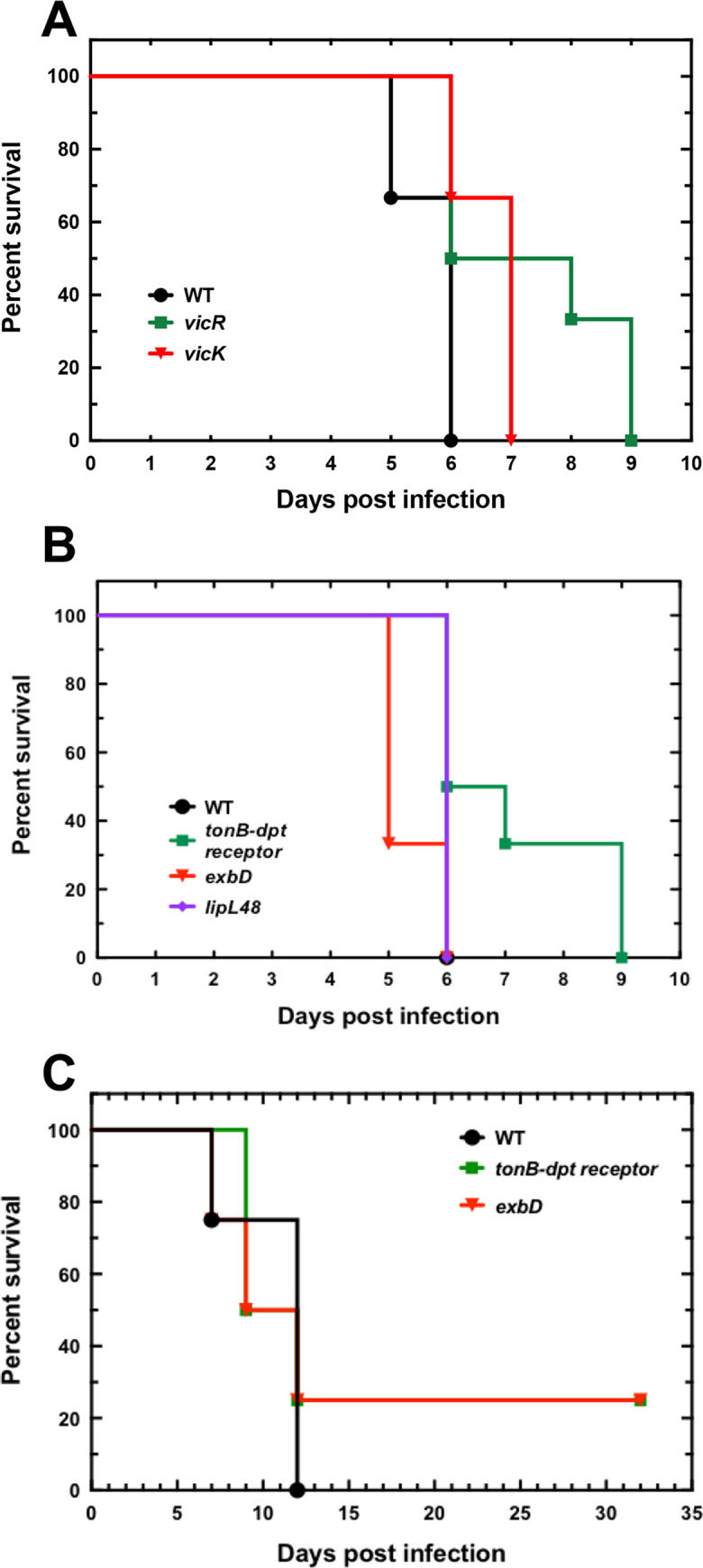
106 of WT, vicK (Man1448) and vicR (Man899) mutant strains (A), or the tonB-dpt receptor (Man1022), exbD (Man782), lipl48 (Man1089) mutant strains (B) or 104 of WT, exbD (Man782), or the tonB-dpt receptor (Man1022) mutant strains (C) were injected intraperitoneally in hamsters (n = 4–8) as described in Material and Methods section.
Discussion
Reactive oxidative species are powerful and efficient weapons used by the host innate immunity response to eliminate pathogens. The ability of pathogenic Leptospira to detoxify hydrogen peroxide, one of the ROS produced upon Leptospira infection and pathogenicity, is essential for these pathogenic bacteria virulence [9]. Because Leptospira are also environmental aerobic bacteria, they will also face low concentrations of ROS endogenously produced through the respiratory chain or present in the outside environment. The present study has used RNA-Seq technology to determine the response of pathogenic Leptospira to hydrogen peroxide. Our study allowed, for the first time, a genome-wide identification of differentially-expressed factors in response to exposure of pathogenic Leptospira to H2O2.
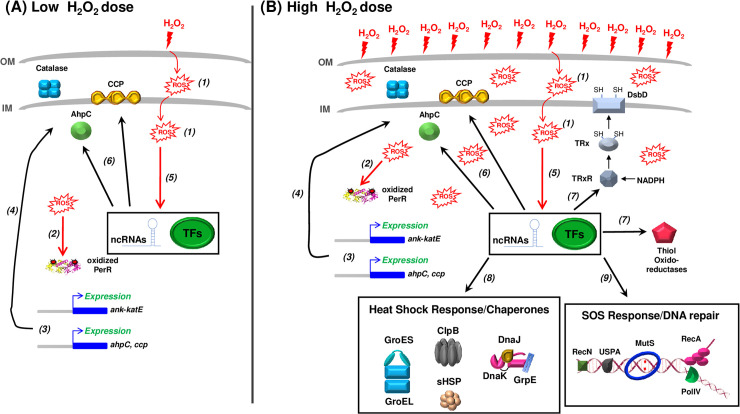
L. interrogans were exposed to sublethal (10 μM) and lethal (1 mM) doses of hydrogen peroxide that could mimic the hydrogen peroxide concentrations encountered inside a host. Our findings indicate that the peroxide stress response is temporal and dose-dependent. L. interrogans can sense and rapidly respond to H2O2 concentrations as low as 10 μM by up-regulating the catalase and two peroxidases, AhpC and CCP (Fig 9A). Heme biosynthesis-encoding genes were also up-regulated probably because catalase and CCP have heme-dependent peroxidase activities. These three peroxidases are the first-line of defense allowing detoxification of H2O2, and among these three enzymes, catalase has a major role in protecting L. interrogans from the deadly effect of hydrogen peroxide, during logarithmic phase but also during stationary phase. Arias et al. [22] showed that E. coli cells overexpressing the L. interrogans AhpC displayed a higher survival in the presence of H2O2 and tert-Butyl hydroperoxide. In our study, an ahpC mutant did not exhibit an altered tolerance toward H2O2; instead, this mutant had a lower ability to grow in the presence of paraquat, a superoxide-generating chemical. Although we cannot rule out that the inactivation of ahpC triggers an increase in catalase activity to compensate the absence of AhpC, our findings might indicate a role of this peroxidase in detoxification of superoxide or of H2O2 produced from the catabolism of superoxide. The role of CCP in degrading H2O2 in pathogenic Leptospira has never been investigated. Whether CCP fulfills such a role or whether CCP rather acts as an electron acceptor for the respiratory chain, as demonstrated in E. coli [34], will require obtaining a deletion mutant by allelic exchange since a ccp mutant was not available in the transposon mutant library.
Fig 9. Schematic representation of the pathogenic Leptospira response to hydrogen peroxide.
In the presence of low H2O2 dose (A), ROS are produced in the cell (1), PerR is oxidized (2) and dissociates from DNA regions in the locus of the three peroxidase-encoding genes (ank-katE operon, ahpC, ccp), leading to their derepression (3) and increased production of catalase, AhpC and CCP (4). Other transcriptional regulator (TFs) and non-coding RNAs whose expression is affected in the presence of ROS (5) probably participate in the H2O2-induced increase of AhpC and CCP production (6). As a result, the activities of catalase, AhpC and CCP allow maintaining ROS at a harmless level. The increased expression of heme biosynthesis genes, which is PerR-independent and probably participates in the peroxidase activities of catalase, AhpC and CCP, is not represented here. When the level of ROS overwhelms the detoxification capacity of the up-regulated peroxidases and becomes damaging for the cellular constituents (B), in addition of a higher production of catalase, AhpC and CCP, other machineries are up-regulated such as thiol oxido-reductases (including thioredoxin, DsbD, etc.) (7), molecular chaperones (8) and DNA repair proteins (9). The increased expression of the aforementioned machineries is PerR-independent and probably involves other transcriptional regulators (TFs) and noncoding RNAs.
The up-regulation of catalase, AhpC and CCP is probably sufficient to rapidly degrade H2O2 and avoid accumulation of ROS inside the cells. However, when the H2O2 level is high, as occurs when L. interrogans are exposed to 1 mM H2O2 (Fig 9B), it could overwhelm the H2O2 detoxification machinery. Additional enzymes with a putative role as antioxidants and/or in repair of oxidized cysteines in proteins are also up-regulated (including thioredoxin, glutaredoxin, DsbD and Bcp-like proteins). The induction of several genes of the LexA regulon (recA, recN, dinP) and other genes with putative role in DNA repair (mutS, radC) suggests that these concentrations of H2O2 induced oxidative damage to DNA and a need for the SOS response. Also, canonical molecular chaperones (DnaK/J/GrpE, GroEL/ES, ClpB, small Hsps) were dramatically more expressed, suggesting that 1 mM H2O2 results in protein aggregation and unfolding.
Several of the factors whose expression is up-regulated upon exposure to H2O2 were also up-regulated when Leptospira are cultivated in DMC implemented in rat peritoneal cavities [16]. Among those were the peroxidases AhpC, CCP, their repressor PerR, RCC1, as well as genes encoding DNA repair proteins (LIMLP_11400, LIMLP_16520, and LIMLP_16525). This suggests that the conditions used in the present study properly reproduce the oxidative stress encountered by Leptospira when infecting a mammalian host. Many H2O2-induced ORFs identified in our study have been shown to be also up-regulated upon other host-related conditions such as at the host temperature of 37°C (Catalase, GroEL/ES, DnaK/J/GrpE, small HSPs, ClpB, RadC) [35–37], host osmolarity (RadC, DsbD, LIMLP_00770, and LIMLP_16520) [38], or under iron-limited condition (LIMLP_08410, LIMLP_14160, Imelysin (LIMLP_14180), and LruB (LIMLP_14170)) [10]. Therefore, the H2O2-induced response overlaps to some extent with other stress responses. In fact, the accumulation of oxidatively-damaged proteins and DNAs could trigger a general stress response. Consistent with this hypothesis is the change in expression of other stress-related regulators such HrcA, LexA, and RpoE.
Comparing the H2O2-induced changes in gene expression in the perR mutant with that in WT cells indicated that PerR contributes only partially to the H2O2-induced gene regulation. Among the genes whose expression is markedly changed upon exposure to H2O2, only katE, ahpC and ccp are under the control of PerR. Surprisingly, even in the absence of PerR, ahpC and ccp expression are still increased upon exposure to H2O2, suggesting that additional regulatory mechanisms are involved in the H2O2-induced gene regulation. In fact, several genes encoding transcriptional regulators, two component systems, and sigma factors had their expression altered by the presence of H2O2, corroborating the involvement of other regulators in the adaptive response to oxidative stress in pathogenic Leptospira (Fig 9B). Moreover, we have identified several ncRNAs that might also influence the expression of the H2O2-regulated genes. Noticeably, rh859 located downstream ccp might participate in the increased expression of this gene, together with the derepression induced by PerR dissociation from DNA in the presence of H2O2 (Fig 9). Therefore, our study has unveiled the complexity of the regulatory network involved in the leptospiral response to oxidative stress.
In the present study, we have further studied the PerR-mediated gene expression control by showing that PerR binds the upstream region of the ank-katE operon, indicating that PerR directly represses this operon. Leptospira PerR was shown in this study to bind more than 1 kb upstream ccp and 500 bp upstream of LIMLP_04285. Such a binding at a distal site from this ORF promoter regions would be consistent with a control of expression mediated by DNA deformation (such as binding or looping) induced by PerR binding. Such a mechanism was demonstrated with the Fur regulator in Helicobacter pylori [39].
Among the ORF that are significantly up-regulated in the presence of H2O2, catalase and ClpB have been shown to be required for Leptospira survival under oxidative stress and virulence [9,37]. In the present study, we have confirmed the essential role of katE for the defense against H2O2, particularly in stationary phase. Furthermore, we have identified new ORFs that participate in Leptospira survival in the presence of ROS. Indeed, our findings indicate that AhpC, a TBDT system, the lipoprotein LipL48, and the response regulator VicR are involved in Leptospira survival in the presence of a superoxide-generating compound. Interestingly, pathogenic Leptospira do not encode any gene homolog to a superoxide dismutase or superoxide reductase, nor they exhibit any SOD activity [40]. This is quite intriguing as it is generally believed that all aerobic bacteria do have a SOD. One fundamental question is to understand the mechanism these pathogenic bacteria use to detoxify superoxide produced endogenously during the respiratory chain or exogenously by phagocytic cells during infection. Our study is the first to identify leptospiral factors in pathogenic Leptospira involved in survival in the presence of superoxide-generating compound. AhpC could detoxify H2O2 produced upon the reduction of superoxide, but the exact function of ExbD, the TBDT, and LipL48 in superoxide detoxification is still unclear. In bacteria, ExbD is part of the inner membrane complex TonB/ExbD/ExbB that uses proton motive force to provide the energy necessary by TonB-dependent transporters for uptake of metal chelates, such as siderophore, or Vitamin B12. The presence of LipL48-encoding ORF in the same operon as the TBDT strongly suggests that these two proteins are functionally linked. This TBDT machinery could be involved in the uptake of metals used by a ROS detoxification enzyme or even acting by themself as ROS scavenger. Indeed, manganese has been shown to scavenge superoxide in Lactobacillus plantarum and Neisseria gonorrhoeae, independently to any SOD activity [41,42].
In conclusion, the present study has revealed, for the first time, the genome-wide general response to peroxide in pathogenic Leptospira, revealing putative biological pathways Leptospira have evolved to overcome the deadly effect of ROS. Peroxide-induced response involves detoxifying enzymes, molecular chaperones and DNA repair machineries. We have also uncovered a complex regulatory network of transcriptional regulators, sigma factors, two component systems and non-coding RNAs that could orchestrate together with PerR the peroxide adaptive response. Peroxide-induced response also engages a large number of non-annotated and sometimes Leptospira-specific ORFs reflecting our limited knowledge on these bacteria physiology.
Materials and methods
Bacterial strains and growth condition
L. interrogans serovar Manilae strain L495 and transposon mutant strains (see S7 Table for a complete description of the transposon mutants used in this study) were grown aerobically at 30°C in Ellinghausen-McCullough-Johnson-Harris medium (EMJH) [43] with shaking at 100 rpm. It should be noted that EMJH medium contains 180 μM FeSO4. Cell growth was followed by measuring the absorbance at 420 nm.
RNA purification
Virulent L. interrogans serovar Manilae strain L495 and perR mutant M776 with less than three in vitro passages were used in this study. Four independent biological replicates of exponentially grown WT and perR mutant L. interrogans strains were incubated in the presence or absence of 10 μM H2O2 for 30 min at 30°C. WT L495 strain was also incubated in the presence of 1 mM H2O2 for 60 min at 30°C. Harvested cells were resuspended in 1 ml TRIzol (ThermoFisher Scientific) and stored at -80°C. Nucleic Acids were extracted with chloroform and precipitated with isopropanol as described elsewhere [44]. Contaminating genomic DNA was removed by DNAse treatment using the RNAse-free Turbo DNA-free turbo kit (ThermoFisher Scientific) as described by the manufacturer. The integrity of RNAs (RIN > 7.6) was verified by the Agilent Bioanalyzer RNA NanoChips (Agilent technologies, Wilmington, DE).
RNA Sequencing
rRNA were depleted from 0.5 μg of total RNA using the Ribo-Zero rRNA Removal Kit (Bacteria) from Illumina. Sequencing libraries were constructed using the TruSeq Stranded mRNA Sample preparation kit (20020595) following the manufacturer’s instructions (Illumina). The directional libraries were controlled on Bioanalyzer DNA1000 Chips (Agilent Technologies) and concentrations measured with the Qubit dsDNA HS Assay Kit (ThermoFisher). Sequences of 65 bases were generated on the Illumina Hiseq 2500 sequencer.
Bioinformatics analyses were performed using the RNA-seq pipeline from Sequana [45]. Reads were cleaned of adapter sequences and low-quality sequences using cutadapt version 1.11 [46]. Only sequences at least 25 nt in length were considered for further analysis. Bowtie version 1.2.2 [47], with default parameters, was used for alignment on the reference genome (L. interrogans serovar Manilae strain UP-MMC-NIID LP, from MicroScope Platform, https://mage.genoscope.cns.fr/microscope/home/index.php). Genes were counted using featureCounts version 1.4.6-p3 [48] from Subreads package (parameters: -t gene -g locus_tag -s 1).
Count data were analyzed using R version 3.5.1 [49] and the Bioconductor package DESeq2 version 1.20.0 [50]. The normalization and dispersion estimation were performed with DESeq2 using the default parameters and statistical tests for differential expression were performed applying the independent filtering algorithm. Differential expressions were expressed as logarithm to base 2 of fold change (Log2FC). A generalized linear model including the replicate effect as blocking factor was set in order to test for the differential expression between Leptospira samples. Raw p-values were adjusted for multiple testing according to the Benjamini and Hochberg (BH) procedure [51] and genes with an adjusted p-value lower than 0.005 and a Log2FC higher than 1 or lower than -1 were considered differentially expressed. The Fisher statistical test was used for the COG (Clusters of Orthologous Groups) classification. Heat maps were generated using the Galaxy platform (https://usegalaxy.eu). The data have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE140019 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE140019).
Quantitative RT-PCR experiments
cDNA synthesis was performed with the cDNA synthesis kit (Biorad) according to the manufacturer’s recommendation. Quantitative PCR was conducted with the SsoFast EvaGreen Supermix (Biorad) as previously described [9,15]. Gene expression was measured with primers described in S8 Table using flaB (LIMLP_09410) as a reference gene.
Non-coding RNA identification
Sequencing data from the Leptospira WT and perR mutant strains incubated in the absence or presence of H2O2 were processed with Trimmomatic [52] to remove low-quality bases and adapter contaminations. BWA mem (version 0.7.12) was used to discard the reads matching Leptospira rRNA, tRNA or polyA sequences and to assign the resulting reads to Leptospira replicons. Then Rockhopper [53] was used to re-align reads corresponding to separate replicons and to assemble transcripts models. The output was filtered to retain all transcripts longer than 50 nucleotides not overlapping within 10 nucleotides with NCBI annotated genes on the same orientation, and showing a minimum Rockhopper raw count value of 50 in at least two isolates. This high-quality set of 778 new sRNAs was subjected to differential expression analysis with Rockhopper, adopting a Benjamini-Hochberg adjusted P-value threshold of 0.01. For each non-coding RNAs, putative function was identified by BLAST using the Rfam database [54].
ChIP-qPCR
Chromatin immunoprecipitation was performed by incubating exponentially growing Leptospira WT or perR1 mutant cells 40 min with 1% formaldehyde at 30°C. The reaction was stopped by the addition of 400 mM glycine. Cells were then washed with TBS buffer and resuspended in buffer A (50 mM HEPES-KOH pH7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100) containing a protease inhibitor cocktail. Cells were sonicated 7 cycles of 15 min. and centrifuged. The supernatant was incubated 3 hours at 4°C with 50 μl of washed Dynabead Pan rabbit IgG. The samples were incubated in the absence or in the presence of anti-PerR serum (at a dilution of 1:750) for 2 hours at 4°C. The samples were successively washed with buffer A containing 500 mM NaCl, with buffer B (10 mM Tris-HCl pH8, 1 mM EDTA, 0.1% Nonidet-P40, 0.5% sodium deoxycholate) and with buffer C (10 mM Tris-HCl pH7.5, 1 mM EDTA). The elution was performed with 100 μl of elution buffer (50 mM Tris-HCl pH7.5, 10 mM EDTA, 1% SDS, 150 mM NaCl, 0.5% Triton X-100) upon an ON incubation at 37°C. An incubation with protease K (2 hours at 65°C) allowed elimination of proteins and DNA fragments were purified. DNA fragments were amplified by qPCR using the indicated primers (see S8 Table) and the SsoFast EvaGreen Supermix (Biorad). Results were normalized by the Fold enrichment method (signal over background) calculated using the following formula: 2^ΔΔCq where ΔΔCq is Cq(with antibody) - Cq(without antibody).
Determination of cell viability
L. interrogans were cultivated in EMJH medium until logarithmic or stationary phase and diluted to ≈ 108/ml. Cells were then incubated in EMJH in the presence or absence of H2O2 for the indicated time. Rezasurin (Alamar Blue Assay, ThermoFisher Scientific) was added and cells were further incubated for 24h. Viability is assessed by the reduction of blue resazurin into pink resorufin [55]. Plating experiments were performed by diluting treated and non-treated cells in EMJH in the absence of H2O2 and plating the samples on EMJH agar medium [55]. Colonies were counted after one-month incubation at 30°C.
Infection experiments
L. interrogans WT and mutant strains were cultivated in EMJH medium until the exponential phase and counted under a dark-field microscope using a Petroff-Hauser cell. 104 or 106 bacteria (in 0.5 ml) were injected intraperitoneally in groups of 4–8 male 4 weeks-old Syrian Golden hamsters (RjHan:AURA, Janvier Labs). Animals were monitored daily and sacrificed by CO2 inhalation when endpoint criteria were met (sign of distress, morbidity).
Ethics statement
The protocol for animal experimentation was reviewed by the Institut Pasteur (Paris, France), the competent authority, for compliance with the French and European regulations on Animal Welfare and with Public Health Service recommendations. This project has been reviewed and approved (CETEA #2016–0019) by the Institut Pasteur ethic committee for animal experimentation, agreed by the French Ministery of Agriculture.
Supporting information
(A) Schematic representation of the ank-katE locus. The DNA fragments amplified by the PCR in (B) are designated with a bar and their corresponding size is indicated in base pairs in parenthesis. The number of nucleotides between different ORFs is indicated in italic. (B) Electrophoresis gels of the PCR-amplified DNA fragments designated in (A) from genomic DNA (gDNA) or from RNA before (RNA) or after (cDNA) a reverse transcriptase reaction. DNA ladder fragment sizes are indicated at left of the gels. (C) Gene expression was measured by RT-qPCR reactions in WT L. interrogans exposed in the absence (white bars) or presence of 10 μM H2O2 for 30 min (black bars) or 2h (dashed bars). Data are mean and SD of three independent experiments. ***, p-value<0.0001 by two-way Anova analysis.
(TIF)
L. interrogans WT cells were cultivated until exponential phase and exposed in the absence (white bars) or presence of 10 μM H2O2 for 30 min (black bars) or for 2h (dashed bars). RNAs were purified and cDNAs were subsequently prepared by reverse transcription. AhpC (A), sufB (A), LIMLP_02790 (B) and ccp (B) expressions were measured by RT-qPCR using flaB (LIMLP_09410) as reference gene and the data were normalized with untreated samples. Data are mean and SD of three independent experiments. ***, p-value<0.0001 by two-way Anova analysis.
(TIF)
(A) Schematic representation of the heme cluster locus. The DNA fragments amplified by the PCR in (B) are designated with a bar and their corresponding size is indicated in base pairs in parenthesis. The number of nucleotides between different ORFs is indicated in italic. (B) Electrophoresis gels of the PCR-amplified DNA fragments designated in (A) from genomic DNA (gDNA) or from RNA before (RNA) or after (cDNA) a reverse transcriptase reaction. DNA ladder fragment sizes are indicated at left of the gels. (C) Gene expression was measured by RT-qPCR reactions in WT L. interrogans exposed in the absence (white bars) or presence of 10 μM H2O2 for 30 min (black bars) or 2h (dashed bars). Data are mean and SD of three independent experiments. ****, p-value<0.0001; **, p-value<0.005; *, p-value<0.05 by two-way Anova analysis.
(TIF)
(A) Schematic representation of the locus of genes coding for a heme oxygenase and a permease. The DNA fragments amplified by the PCR in (B) are designated with a bar and their corresponding size is indicated in base pairs in parenthesis. The number of nucleotides between different ORFs is indicated in italic. (B) Electrophoresis gels of the PCR-amplified DNA fragments designated in (A) from genomic DNA (gDNA) or from RNA before (RNA) or after (cDNA) a reverse transcriptase reaction. DNA ladder fragment sizes are indicated at left of the gels. (C) Gene expression was measured by RT-qPCR reactions in WT L. interrogans exposed in the absence (white bars) or presence of 10 μM H2O2 for 30 min (black bars) or 2h (dashed bars). Data are mean and SD of three independent experiments. *, p-value<0.05 by two-way Anova analysis.
(TIF)
Chromatin immunoprecipitation was performed on L. interrogans WT and perR (M776) mutant strains in the presence or absence of the anti-PerR antibody. Co-immunoprecipitated DNA fragments located in the ank-katE operon locus (A), in the ccp locus (B) and in the ahpC locus (C) were amplified by qPCR. The location of amplified fragments is indicated below the schematic representation of their respective loci. The number of nucleotides between different ORFs is indicated in italic. Data are represented as fold enrichments.
(TIF)
(A) Schematic representation of the locus of genes coding for a TonB-dependent transport system. The DNA fragments amplified by the PCR in (B) are designated with a bar and their corresponding size is indicated in base pairs in parenthesis. The number of nucleotides between different ORFs is indicated in italic. (B) Electrophoresis gels of the PCR-amplified DNA fragments designated in (A) from genomic DNA (gDNA) or from RNA before (RNA) or after (cDNA) a reverse transcriptase reaction. DNA ladder fragment sizes are indicated at left of the gels.
(TIF)
Chromatin immunoprecipitation was performed on L. interrogans WT and perR (M776) mutant strains in the presence or absence of the anti-PerR antibody. Co-immunoprecipitated DNA fragments located in the locus encoding a TonB-dependent transporter system were amplified by qPCR. The location of amplified fragments is indicated below the schematic representation of the locus. The number of nucleotides between different ORFs is indicated in italic. Data are represented as fold enrichments.
(TIF)
(A) Schematic representation of the locus of genes coding for the histidine kinase VicK and the response regulator VicR. The DNA fragments amplified by the PCR in (B) are designated with a bar and their corresponding size is indicated in base pairs in parenthesis. The number of nucleotides between different ORFs is indicated in italic. (B) Electrophoresis gels of the PCR-amplified DNA fragments designated in (A) from genomic DNA (gDNA) or from RNA before (RNA) or after (cDNA) a reverse transcriptase reaction. DNA ladder fragment sizes are indicated at left of the gels.
(TIF)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(DOCX)
(XLSX)
Acknowledgments
We would like to thank Maya Long and Clémence Mouville for their excellent and efficient technical help. CZA is part of the Pasteur-Paris University (PPU) International PhD Program. We also would like to thank the Amgen Foundation and Scholars Program for supporting JB and SGH.
Data Availability
RNA Sequencing data have been deposited at the NCBI Gene Expression Omnibus (GEO) under the accession number GSE140019 (http://www.ncbi.nlm.nih.gov/geo).
Funding Statement
CZA was awarded a grant from the Fondation Etchèbes-Fondation de France (S-CM16008) (https://www.fondationdefrance.org/fr/fondation/fondation-etchebes). This project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 665807. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65–97. 10.1007/978-3-662-45059-8_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl Trop Dis. 2015. September 17;9(9):e0003898–e0003898. 10.1371/journal.pntd.0003898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pijnacker R, Goris MGA, te Wierik MJM, Broens EM, van der Giessen JWB, de Rosa M, et al. Marked increase in leptospirosis infections in humans and dogs in the Netherlands, 2014. Eurosurveillance [Internet]. 2016;21(17). Available from: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2016.21.17.30211 [DOI] [PubMed] [Google Scholar]
- 4.Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009. October;7(10):736–47. 10.1038/nrmicro2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picardeau M. Virulence of the zoonotic agent of leptospirosis: still terra incognita? Nat Rev Microbiol. 2017;15(5):297–307. 10.1038/nrmicro.2017.5 [DOI] [PubMed] [Google Scholar]
- 6.Marangoni A, Accardo S, Aldini R, Guardigli M, Cavrini F, Sambri V, et al. Production of reactive oxygen species and expression of inducible nitric oxide synthase in rat isolated Kupffer cells stimulated by Leptospira interrogans and Borrelia burgdorferi. World J Gastroenterol. 2006. May 21;12(19):3077–81. 10.3748/wjg.v12.i19.3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araújo AM, Reis EAG, Athanazio DA, Ribeiro GS, Hagan JE, Araujo GC, et al. Oxidative stress markers correlate with renal dysfunction and thrombocytopenia in severe leptospirosis. Am J Trop Med Hyg. 2014. April;90(4):719–23. 10.4269/ajtmh.13-0667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdogan HM, Karapehlivan M, Citil M, Atakisi O, Uzlu E, Unver A. Serum sialic acid and oxidative stress parameters changes in cattle with leptospirosis. Veterinary Research Communications. 2008. April 1;32(4):333–9. 10.1007/s11259-008-9036-z [DOI] [PubMed] [Google Scholar]
- 9.Eshghi A, Lourdault K, Murray GL, Bartpho T, Sermswan RW, Picardeau M, et al. Leptospira interrogans Catalase Is Required for Resistance to H2O2 and for Virulence. Blanke SR, editor. Infect Immun. 2012. November 1;80(11):3892 10.1128/IAI.00466-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo M, Murray GL, Khoo CA, Haake DA, Zuerner RL, Adler B. Transcriptional response of Leptospira interrogans to iron limitation and characterization of a PerR homolog. Infect Immun. 2010. November;78(11):4850–9. 10.1128/IAI.00435-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faulkner MJ, Helmann JD. Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxid Redox Signal. 2011. July 1;15(1):175–89. 10.1089/ars.2010.3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacquamet L, Traoré D a. K, Ferrer J-L, Proux O, Testemale D, Hazemann J-L, et al. Structural characterization of the active form of PerR: insights into the metal-induced activation of PerR and Fur proteins for DNA binding. Mol Microbiol. 2009. July;73(1):20–31. 10.1111/j.1365-2958.2009.06753.x [DOI] [PubMed] [Google Scholar]
- 13.Lee J-W, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006. March 16;440(7082):363–7. 10.1038/nature04537 [DOI] [PubMed] [Google Scholar]
- 14.Traoré DAK, El Ghazouani A, Jacquamet L, Borel F, Ferrer J-L, Lascoux D, et al. Structural and functional characterization of 2-oxo-histidine in oxidized PerR protein. Nat Chem Biol. 2009. January;5(1):53–9. 10.1038/nchembio.133 [DOI] [PubMed] [Google Scholar]
- 15.Kebouchi M, Saul F, Taher R, Landier A, Beaudeau B, Dubrac S, et al. Structure and function of the Leptospira interrogans peroxide stress regulator (PerR), an atypical PerR devoid of a structural metal-binding site. J Biol Chem. 2018. January 12;293(2):497–509. 10.1074/jbc.M117.804443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caimano MJ, Sivasankaran SK, Allard A, Hurley D, Hokamp K, Grassmann AA, et al. A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Leptospira interrogans Serovar Copenhageni. PLOS Pathogens. 2014. March 13;10(3):e1004004 10.1371/journal.ppat.1004004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satou K, Shimoji M, Tamotsu H, Juan A, Ashimine N, Shinzato M, et al. Complete Genome Sequences of Low-Passage Virulent and High-Passage Avirulent Variants of Pathogenic Leptospira interrogans Serovar Manilae Strain UP-MMC-NIID, Originally Isolated from a Patient with Severe Leptospirosis, Determined Using PacBio Single-Molecule Real-Time Technology. Genome Announc. 2015. August 13;3(4):e00882–15. 10.1128/genomeA.00882-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faine S. Catalase activity in pathogenic Leptospira. J Gen Microbiol. 1960. February;22:1–9. 10.1099/00221287-22-1-1 [DOI] [PubMed] [Google Scholar]
- 19.Rao PJ, Larson A D AD, Cox C D CD. Catalase activity in Leptospira. J Bacteriol. 1964. October;88(4):1045–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flint A, Stintzi A. Cj1386, an atypical hemin-binding protein, mediates hemin trafficking to KatA in Campylobacter jejuni. J Bacteriol. 2015. March;197(5):1002–11. 10.1128/JB.02346-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howell ML, Alsabbagh E, Ma JF, Ochsner UA, Klotz MG, Beveridge TJ, et al. AnkB, a periplasmic ankyrin-like protein in Pseudomonas aeruginosa, is required for optimal catalase B (KatB) activity and resistance to hydrogen peroxide. J Bacteriol. 2000. August;182(16):4545–56. 10.1128/jb.182.16.4545-4556.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arias DG, Reinoso A, Sasoni N, Hartman MD, Iglesias AA, Guerrero SA. Kinetic and structural characterization of a typical two-cysteine peroxiredoxin from Leptospira interrogans exhibiting redox sensitivity. Free Radic Biol Med. 2014. December;77:30–40. 10.1016/j.freeradbiomed.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 23.Jensen LMR, Sanishvili R, Davidson VL, Wilmot CM. In crystallo posttranslational modification within a MauG/pre-methylamine dehydrogenase complex. Science. 2010. March 12;327(5971):1392–4. 10.1126/science.1182492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guégan R, Camadro J-M, Saint Girons I, Picardeau M. Leptospira spp. possess a complete haem biosynthetic pathway and are able to use exogenous haem sources. Mol Microbiol. 2003. August;49(3):745–54. 10.1046/j.1365-2958.2003.03589.x [DOI] [PubMed] [Google Scholar]
- 25.Zhukova A, Fernandes LG, Hugon P, Pappas CJ, Sismeiro O, Coppée J-Y, et al. Genome-Wide Transcriptional Start Site Mapping and sRNA Identification in the Pathogen Leptospira interrogans. Front Cell Infect Microbiol. 2017;7:10 10.3389/fcimb.2017.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verma A, Kumar P, Babb K, Timoney JF, Stevenson B. Cross-reactivity of antibodies against leptospiral recurrent uveitis-associated proteins A and B (LruA and LruB) with eye proteins. PLoS Negl Trop Dis. 2010. August 3;4(8):e778 10.1371/journal.pntd.0000778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasoni N, Iglesias AA, Guerrero SA, Arias DG. Functional thioredoxin reductase from pathogenic and free-living Leptospira spp. Free Radic Biol Med. 2016;97:1–13. 10.1016/j.freeradbiomed.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 28.Fonseca LS, da Silva JB, Milanez JS, Monteiro-Vitorello CB, Momo L, de Morais ZM, et al. Leptospira interrogans serovar copenhageni harbors two lexA genes involved in SOS response. PLoS ONE. 2013;8(10):e76419 10.1371/journal.pone.0076419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schons-Fonseca L, da Silva JB, Milanez JS, Domingos RH, Smith JL, Nakaya HI, et al. Analysis of LexA binding sites and transcriptomics in response to genotoxic stress in Leptospira interrogans. Nucleic Acids Res. 2016. February 18;44(3):1179–91. 10.1093/nar/gkv1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin J-H, Zhang Q, Zhang Z-M, Zhong Y, Yang Y, Hu B-Y, et al. Identification of a novel prophage-like gene cluster actively expressed in both virulent and avirulent strains of Leptospira interrogans serovar Lai. Infect Immun. 2008. June;76(6):2411–9. 10.1128/IAI.01730-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourhy P, Louvel H, Saint Girons I, Picardeau M. Random Insertional Mutagenesis of Leptospira interrogans, the Agent of Leptospirosis, Using a mariner Transposon. J Bacteriol. 2005. May 1;187(9):3255–8. 10.1128/JB.187.9.3255-3258.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bol DK, Yasbin RE. Analysis of the dual regulatory mechanisms controlling expression of the vegetative catalase gene of Bacillus subtilis. J Bacteriol. 1994. November;176(21):6744–8. 10.1128/jb.176.21.6744-6748.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Visick JE, Clarke S. RpoS- and OxyR-independent induction of HPI catalase at stationary phase in Escherichia coli and identification of rpoS mutations in common laboratory strains. J Bacteriol. 1997. July;179(13):4158–63. 10.1128/jb.179.13.4158-4163.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khademian M, Imlay JA. Escherichia coli cytochrome c peroxidase is a respiratory oxidase that enables the use of hydrogen peroxide as a terminal electron acceptor. Proc Natl Acad Sci U S A. 2017. August 15;114(33):E6922–31. 10.1073/pnas.1701587114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo M, Bulach DM, Powell DR, Haake DA, Matsunaga J, Paustian ML, et al. Effects of temperature on gene expression patterns in Leptospira interrogans serovar Lai as assessed by whole-genome microarrays. Infect Immun. 2006. October;74(10):5848–59. 10.1128/IAI.00755-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo M, Cordwell SJ, Bulach DM, Adler B. Comparative transcriptional and translational analysis of leptospiral outer membrane protein expression in response to temperature. PLoS Negl Trop Dis. 2009. December 8;3(12):e560–e560. 10.1371/journal.pntd.0000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lourdault K, Cerqueira GM, Wunder EA, Picardeau M. Inactivation of clpB in the pathogen Leptospira interrogans reduces virulence and resistance to stress conditions. Infect Immun. 2011. September;79(9):3711–7. 10.1128/IAI.05168-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsunaga J, Lo M, Bulach DM, Zuerner RL, Adler B, Haake DA. Response of Leptospira interrogans to physiologic osmolarity: relevance in signaling the environment-to-host transition. Infect Immun. 2007. June;75(6):2864–74. 10.1128/IAI.01619-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roncarati D, Pelliciari S, Doniselli N, Maggi S, Vannini A, Valzania L, et al. Metal-responsive promoter DNA compaction by the ferric uptake regulator. Nature Communications. 2016. August 25;7(1):12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin FE, Barbieri JT, Corin RE, Grigas KE, Cox CD. Distribution of superoxide dismutase, catalase, and peroxidase activities among Treponema pallidum and other spirochetes. Infect Immun. 1981. August;33(2):372–9. 10.1128/IAI.33.2.372-379.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Archibald FS, Fridovich I. The scavenging of superoxide radical by manganous complexes: In vitro. Archives of Biochemistry and Biophysics. 1982. April 1;214(2):452–63. 10.1016/0003-9861(82)90049-2 [DOI] [PubMed] [Google Scholar]
- 42.Tseng H-J, Srikhanta Y, McEwan AG, Jennings MP. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Molecular Microbiology. 2001. June 1;40(5):1175–86. 10.1046/j.1365-2958.2001.02460.x [DOI] [PubMed] [Google Scholar]
- 43.Ellinghausen HC, Mccullough WG. Nutrition of Leptospira pomona and growth of 13 other serotypes: a serum-free medium employing oleic albumin complex. Am J Vet Res. 1965. January;26:39–44. [PubMed] [Google Scholar]
- 44.Zavala-Alvarado C, Benaroudj N. The Single-Step Method of RNA Purification Applied to Leptospira. Methods Mol Biol. 2020;2134:41–51. [DOI] [PubMed] [Google Scholar]
- 45.Cokelaer T, Desvillechabrol D, Legendre R, Cardon M. ‘Sequana’: a Set of Snakemake NGS pipelines. The Journal of Open Source Software. 2(16). [Google Scholar]
- 46.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal; Vol 17, No 1: Next Generation Sequencing Data AnalysisDO—1014806/ej171200 [Internet]. 2011 May 2; Available from: http://journal.embnet.org/index.php/embnetjournal/article/view/200
- 47.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biology. 2009. March 4;10(3):R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2013. November 13;30(7):923–30. 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- 49.R core Team. R, a language and environment for statistcial computing [Internet]. 2016. Available from: https://www.gbif.org/en/tool/81287/r-a-language-and-environment-for-statistical-computing
- 50.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014. December 5;15(12):550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 52.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014. August 1;30(15):2114–20. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McClure R, Balasubramanian D, Sun Y, Bobrovskyy M, Sumby P, Genco CA, et al. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res. 2013. August;41(14):e140–e140. 10.1093/nar/gkt444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalvari I, Argasinska J, Quinones-Olvera N, Nawrocki EP, Rivas E, Eddy SR, et al. Rfam 13.0: shifting to a genome-centric resource for non-coding RNA families. Nucleic Acids Research. 2017. November 3;46(D1):D335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mouville C, Benaroudj N. Survival Tests for Leptospira spp. Methods Mol Biol. 2020;2134:215–28. 10.1007/978-1-0716-0459-5_20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Schematic representation of the ank-katE locus. The DNA fragments amplified by the PCR in (B) are designated with a bar and their corresponding size is indicated in base pairs in parenthesis. The number of nucleotides between different ORFs is indicated in italic. (B) Electrophoresis gels of the PCR-amplified DNA fragments designated in (A) from genomic DNA (gDNA) or from RNA before (RNA) or after (cDNA) a reverse transcriptase reaction. DNA ladder fragment sizes are indicated at left of the gels. (C) Gene expression was measured by RT-qPCR reactions in WT L. interrogans exposed in the absence (white bars) or presence of 10 μM H2O2 for 30 min (black bars) or 2h (dashed bars). Data are mean and SD of three independent experiments. ***, p-value<0.0001 by two-way Anova analysis.
(TIF)
L. interrogans WT cells were cultivated until exponential phase and exposed in the absence (white bars) or presence of 10 μM H2O2 for 30 min (black bars) or for 2h (dashed bars). RNAs were purified and cDNAs were subsequently prepared by reverse transcription. AhpC (A), sufB (A), LIMLP_02790 (B) and ccp (B) expressions were measured by RT-qPCR using flaB (LIMLP_09410) as reference gene and the data were normalized with untreated samples. Data are mean and SD of three independent experiments. ***, p-value<0.0001 by two-way Anova analysis.
(TIF)
(A) Schematic representation of the heme cluster locus. The DNA fragments amplified by the PCR in (B) are designated with a bar and their corresponding size is indicated in base pairs in parenthesis. The number of nucleotides between different ORFs is indicated in italic. (B) Electrophoresis gels of the PCR-amplified DNA fragments designated in (A) from genomic DNA (gDNA) or from RNA before (RNA) or after (cDNA) a reverse transcriptase reaction. DNA ladder fragment sizes are indicated at left of the gels. (C) Gene expression was measured by RT-qPCR reactions in WT L. interrogans exposed in the absence (white bars) or presence of 10 μM H2O2 for 30 min (black bars) or 2h (dashed bars). Data are mean and SD of three independent experiments. ****, p-value<0.0001; **, p-value<0.005; *, p-value<0.05 by two-way Anova analysis.
(TIF)
(A) Schematic representation of the locus of genes coding for a heme oxygenase and a permease. The DNA fragments amplified by the PCR in (B) are designated with a bar and their corresponding size is indicated in base pairs in parenthesis. The number of nucleotides between different ORFs is indicated in italic. (B) Electrophoresis gels of the PCR-amplified DNA fragments designated in (A) from genomic DNA (gDNA) or from RNA before (RNA) or after (cDNA) a reverse transcriptase reaction. DNA ladder fragment sizes are indicated at left of the gels. (C) Gene expression was measured by RT-qPCR reactions in WT L. interrogans exposed in the absence (white bars) or presence of 10 μM H2O2 for 30 min (black bars) or 2h (dashed bars). Data are mean and SD of three independent experiments. *, p-value<0.05 by two-way Anova analysis.
(TIF)
Chromatin immunoprecipitation was performed on L. interrogans WT and perR (M776) mutant strains in the presence or absence of the anti-PerR antibody. Co-immunoprecipitated DNA fragments located in the ank-katE operon locus (A), in the ccp locus (B) and in the ahpC locus (C) were amplified by qPCR. The location of amplified fragments is indicated below the schematic representation of their respective loci. The number of nucleotides between different ORFs is indicated in italic. Data are represented as fold enrichments.
(TIF)
(A) Schematic representation of the locus of genes coding for a TonB-dependent transport system. The DNA fragments amplified by the PCR in (B) are designated with a bar and their corresponding size is indicated in base pairs in parenthesis. The number of nucleotides between different ORFs is indicated in italic. (B) Electrophoresis gels of the PCR-amplified DNA fragments designated in (A) from genomic DNA (gDNA) or from RNA before (RNA) or after (cDNA) a reverse transcriptase reaction. DNA ladder fragment sizes are indicated at left of the gels.
(TIF)
Chromatin immunoprecipitation was performed on L. interrogans WT and perR (M776) mutant strains in the presence or absence of the anti-PerR antibody. Co-immunoprecipitated DNA fragments located in the locus encoding a TonB-dependent transporter system were amplified by qPCR. The location of amplified fragments is indicated below the schematic representation of the locus. The number of nucleotides between different ORFs is indicated in italic. Data are represented as fold enrichments.
(TIF)
(A) Schematic representation of the locus of genes coding for the histidine kinase VicK and the response regulator VicR. The DNA fragments amplified by the PCR in (B) are designated with a bar and their corresponding size is indicated in base pairs in parenthesis. The number of nucleotides between different ORFs is indicated in italic. (B) Electrophoresis gels of the PCR-amplified DNA fragments designated in (A) from genomic DNA (gDNA) or from RNA before (RNA) or after (cDNA) a reverse transcriptase reaction. DNA ladder fragment sizes are indicated at left of the gels.
(TIF)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(DOCX)
(XLSX)
Data Availability Statement
RNA Sequencing data have been deposited at the NCBI Gene Expression Omnibus (GEO) under the accession number GSE140019 (http://www.ncbi.nlm.nih.gov/geo).