Figure 5.
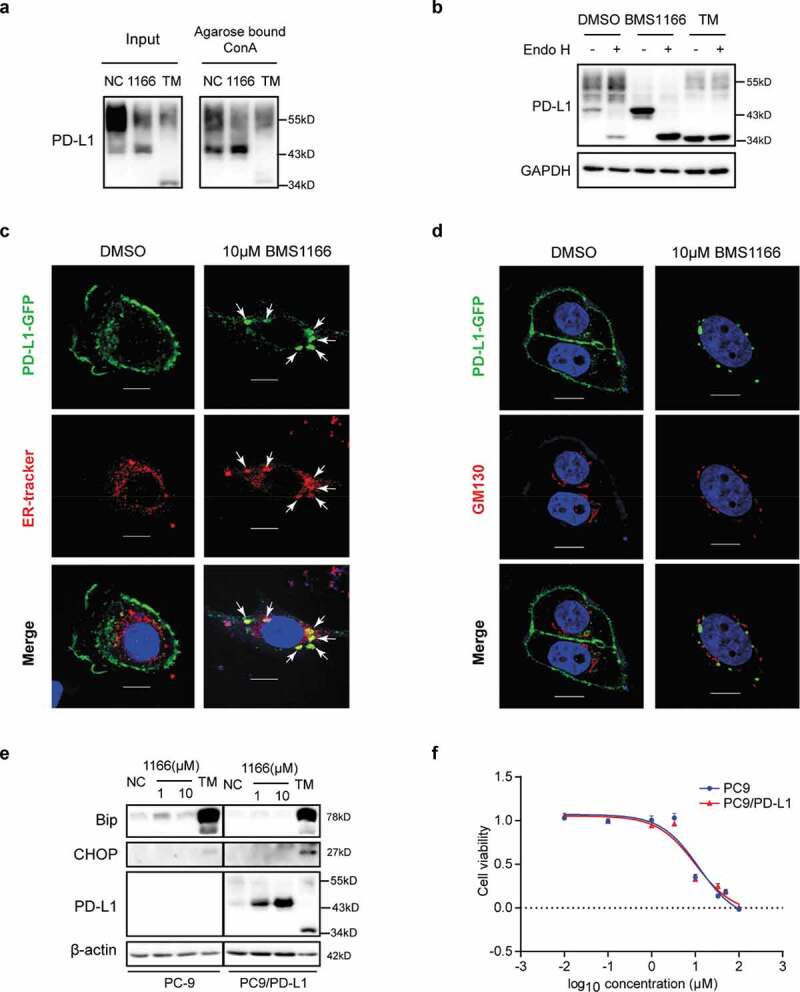
BMS1166 retained PD-L1 in ER and prevented its further glycosylation without inducing ER stress. (a) PC9/PD-L1 cells were treated with DMSO, 10 μM BMS1166 or 1 μg/ml TM for 17 hr, and the whole cell lysates were collected as “input”, and part of the lysates were incubated with agarose bound with Con A overnight as “Agarose bound Con A”. All the samples were processed to western blot analysis using antibodies as indicated. (b) PC9/PD-L1 cells were treated with DMSO, 10 μM BMS1166 or 1 μg/ml TM for 17 hr, and the whole cell lysates were collected and incubated with or without Endo H enzyme for 1 hr at 37°C. All the samples were processed to western blot analysis using antibodies as indicated. The anti-GAPDH antibody was used as a control for equal protein loading. (c) and (d) PC-9 cells were transfected with PD-L1-GFP plasmids for 24 hr and then treated with DMSO or 10 μM BMS1166 for 17 hr. Fixed samples were stained with ER-tracker red (1:1000) (c) or anti-GM130 (d) and 0.1 μg/ml DAPI, and then were visualized by confocal microscopy. White arrows point to co-localization of PD-L1-GFP and ER-tracker. Bar, 10 μm. (e) PC-9 or PC9/PD-L1 cells were incubated with DMSO (NC), BMS1166 or 1 μg/ml TM for 17 hr. Whole cell lysates were processed for western blotting analysis using antibodies as indicated. Anti-β-actin antibody was used as a control for equal protein loading. (f) PC-9 or PC9/PD-L1 cells were incubated with indicated concentrations of BMS1166 for 48 hr. Cell viability was assessed by MTT assay.
