Fig. 5. EGFR and PI3K/PTEN differentially activate CAD phosphorylation sites.
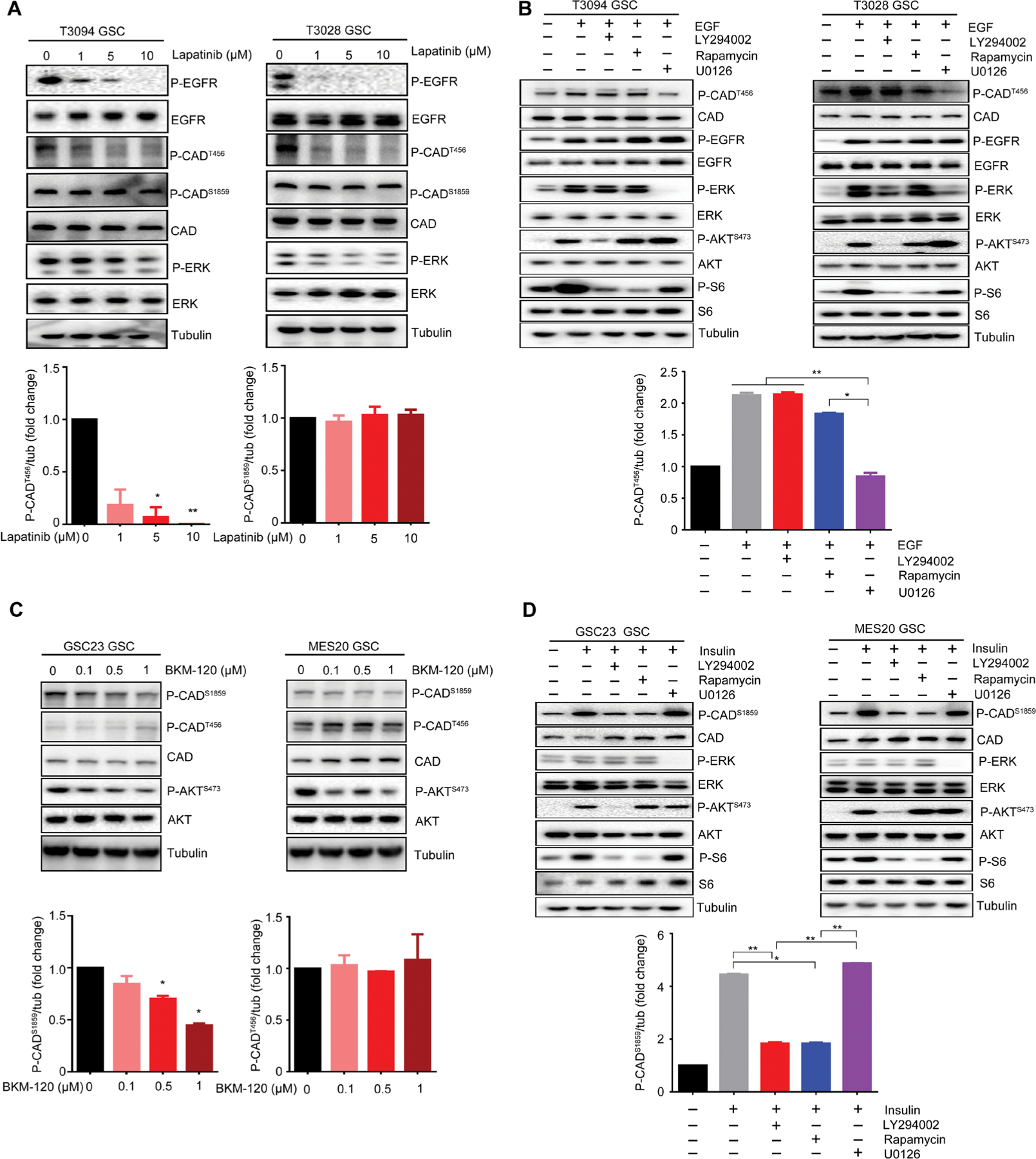
(A) Immunoblot assessment of phosphorylated EGFR, phosphorylated ERK, phosphorylated CADS1859, phosphorylated CADT456, and total CAD protein after treatment with the EGFR inhibitor lapatinib (1, 5, and 10 μM) for 48 hours in T3094 and T3028 GSCs. Normalized quantifications with tubulin as control were performed for phosphorylated CADS1859 and phosphorylated CADT456 (n = 2 biological replicates; *P < 0.05 and **P < 0.01, one-way ANOVA). (B) GSCs (T3094 and T3028) were treated with EGF (100 ng/ml) over a 30-min time course, together with inhibitors of the PI3K (LY294002), mTOR (rapamycin), or MEK (U0126) pathways. Amounts of phosphorylated CADT456, total CAD, phosphorylated EGFR, total EGFR, phosphorylated ERK, total ERK, phosphorylated AKTS473, total AKT, phosphorylated S6, and total S6 were assessed by immunoblot. Normalized quantifications with tubulin as control were performed for phosphorylated CADT456 (n = 2 biological replicates; *P < 0.05 and **P < 0.01, one-way ANOVA). (C) Immunoblot assessment of phosphorylated CADS1859, phosphorylated CADT456, total CAD, phosphorylated AKTS473, and total AKT after treatment with the PI3K inhibitor BKM120 (0.1, 0.5, and 1 μM) for 48 hours in GSC23 and MES20 GSCs. Normalized quantifications with tubulin as control were performed for phosphorylated CADS1859 and phosphorylated CADT456 (n = 2 biological replicates; *P < 0.05, one-way ANOVA). (D) GSCs (GSC23 and MES20) were treated with insulin (1 μM) over a 30-minute time course, together with inhibitors of the PI3K (LY294002), mTOR (rapamycin), or MEK (U0126) pathways. Amounts of phosphorylated CADS1859, total CAD, phosphorylated ERK, total ERK, phosphorylated AKTS473, total AKT, phosphorylated S6, and total S6 were assessed by immunoblot. Normalized quantifications with tubulin as control were performed for phosphorylated CADS1859 (n = 2 biological repli cates; *P < 60.05 and **P < 0.01, one-way ANOVA).
