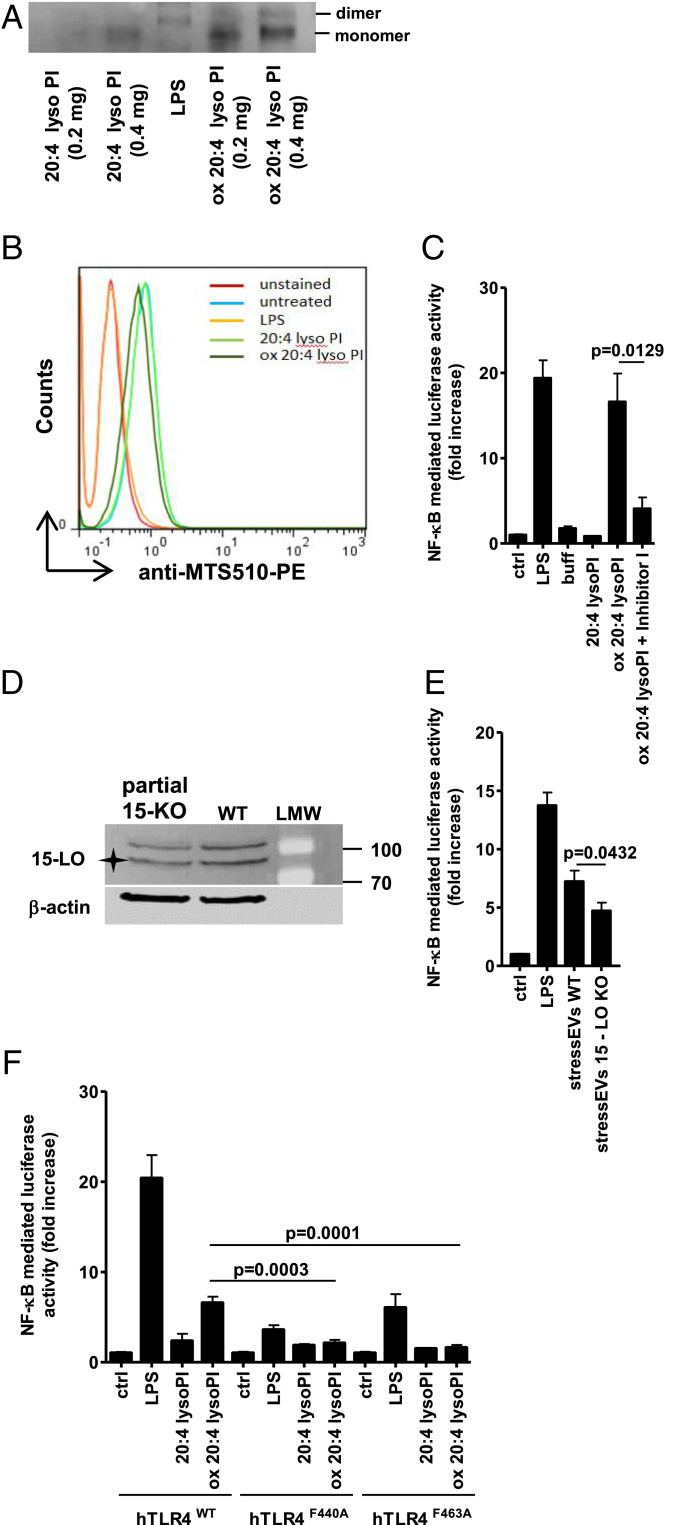
Fig. 4.
Mechanism of oxidized PL formation and TLR4 signaling. (A) Indicated amounts of 20:4 lysoPI or oxidized 20:4 lysoPI were incubated with TLR4/MD-2 recombinant protein (0.5 μg) and then samples were loaded to 10% native PAGE. The dimerization of TLR4/MD-2 was analyzed by Western blot. (B) RAW 264.7 cells were stimulated with 20:4 lysoPI, oxidized 20:4 lysoPI (40 μg/mL), or LPS (1 μg/mL) as a positive control for 6 h. TLR4/MD-2 dimerization using anti-MTS510-PE Ab was determined by flow cytometry. (C and E) HEK293T cells expressing hTLR4, hMD-2/CD14, as well as firefly luciferase under NF-κB promotor and Renilla luciferase for normalization were stimulated with 20:4 lysoPI (5 μg/mL), oxidized 20:4 lysoPI (5 μg/mL) in the presence or absence of Inhibitor I (1 mM) in C, stressEVs WT (5 μg/mL), stressEVs 15-LO KO (5 μg/mL) in E or LPS (10 ng/mL) as a positive control for 24 h. Dual luciferase tests for NF-κB activity were performed. (D) Western blot detection of 15-LO and β-actin (loading control) in WT and partial 15-LO KO HEK293 cells. Asterisk indicates specific band. (F) HEK293T cells expressing hMD-2/CD14 and hTLR4WT, hTLR4F440A, or hTLR4F463A as well as luciferase under NF-κB promotor and Renilla luciferase for normalization were stimulated with 20:4 lysoPI (5 μg/mL), oxidized 20:4 lysoPI (5 μg/mL), or LPS (10 ng/mL) as a positive control for 24 h. Dual luciferase test for NF-κB activity was performed. (C) Two indep. exps. (n = 6); t test (two-tailed; unequal var.); (E) three indep. exps. (n = 6 or 12); t test (two-tailed; unequal var.); (F) three indep. exps. (n = 8); t test (two-tailed; unequal var.). For B an additional indep. exp. is shown in SI Appendix, Additional data.