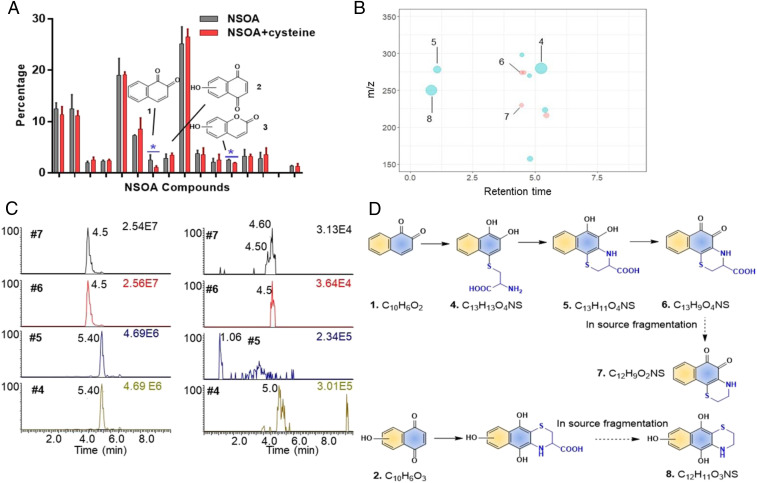
Fig. 6.
Nontargeted identification of unsaturated carbonyls as the cysteine reactive components. (A) Percentage of the 16 high abundant constituents of NSOA before and after incubation with cysteine. Only the abundances of chemical 1 and 3 were reduced significantly. (B) Cysteine adducts were detected after incubation with cysteine. The size of each dot is proportional to the peak intensity. Red represents peaks detected under ESI−, while blue represents peaks detected under ESI+. The x axis represents the retention time of cysteine adducts on C18 column in UPLC. (C) Authentic standard was used to confirm chemicals 4–7 as the cysteine adducts of 1,2-naphthoquinone (rt = 4.50 min for adduct 7) and 1,4-naphthoquinone (rt = 4.60 min for adduct 7). Left panel shows the chromatograms of cysteine adducts of 1,2-naphthequinone from authentic standards (10 µM). Right panel shows the chromatograms of cysteine adducts of 1,2-naphthequinone NSOA. Notice that adducts 4 and 5 were detected from authentic standards with the same m/z, due to the oxidation during ionization process under ESI+. (D) Proposed reaction pathways of unsaturated carbonyls 1 and 2 with cysteine.