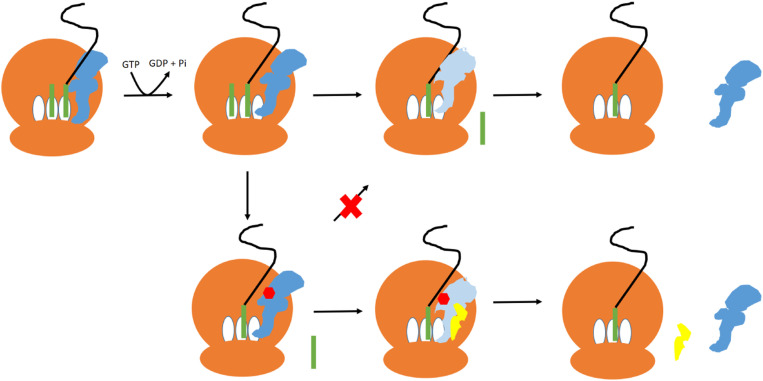
Fig. 5.
The proposed mechanism of FusB-mediated FA resistance. In the absence of FA, EF-G (blue) binds to the ribosome (brown) and catalyzes translocation of the tRNA (green) from the A (acceptor) and P (peptidyl) to the P and E (exit) sites. GTP hydrolysis causes EF-G to undergo a conformational change (light blue), allowing EF-G to dissociate from the ribosome. In the presence of FA, FA (red) binds to EF-G following GTP hydrolysis and prevents the conformational change so that EF-G does not dissociate from the ribosome. FusB (yellow) binding to EF-G promotes an increased population of a minor state of EF-G with a more disordered domain III, allowing EF-G to undergo the conformational change required for release from the ribosome even while bound to FA.