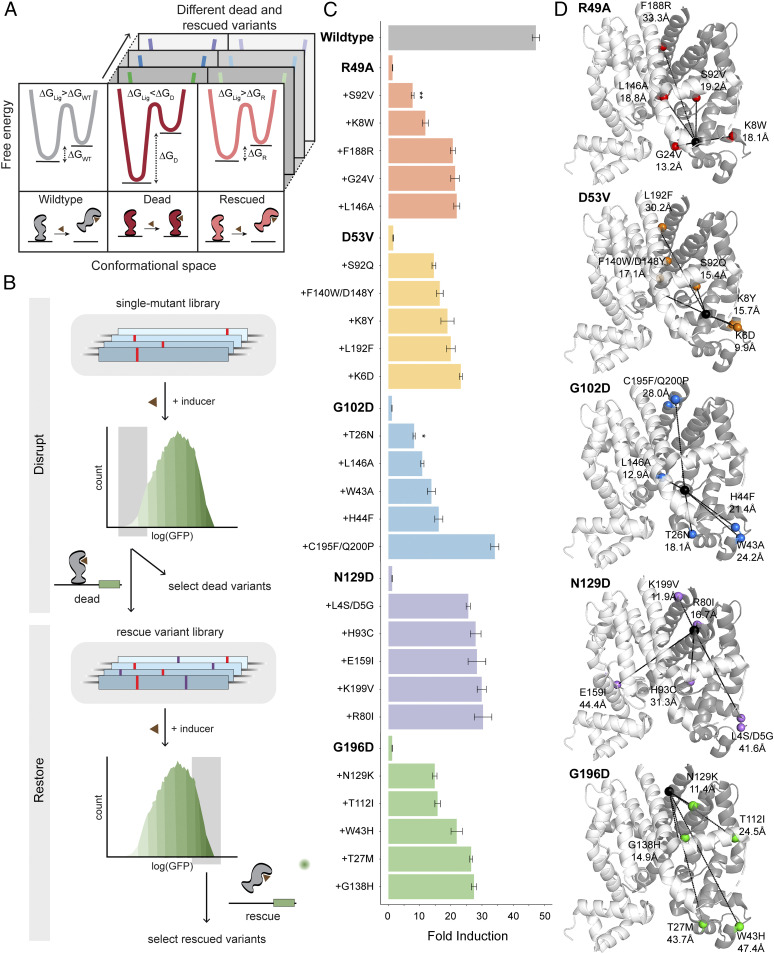
Fig. 1.
”Disrupt-and-restore” strategy to characterize plasticity of TetR allostery. (A) A simplified thermodynamic model of allosteric signaling (detailed model SI Appendix, Fig. S2). (Left) Ligand binding provides the driving force to switch from inactive to active state in wild type. (Center) A disrupting mutation (dead variant) makes TetR constitutively inactive due to larger energy difference. (Right) Allosteric function is restored (rescued variant) by energetic rebalancing via distal compensatory mutations. (B) Two-stage protein-wide mutational scanning to first disrupt and subsequently restore allosteric signaling using a GFP-based transcriptional reporter system. (C) Fold induction of individual variants (mean ± SEM) in the presence in 1 µM aTC of three biological replicates. All rescue variants are significantly different from the corresponding dead (P < 0.001 unless noted; *P < 0.05, **P < 0.01). (D) Five dead variants (black spheres), their corresponding rescued variants (colored spheres), and distances between them (dashed lines).