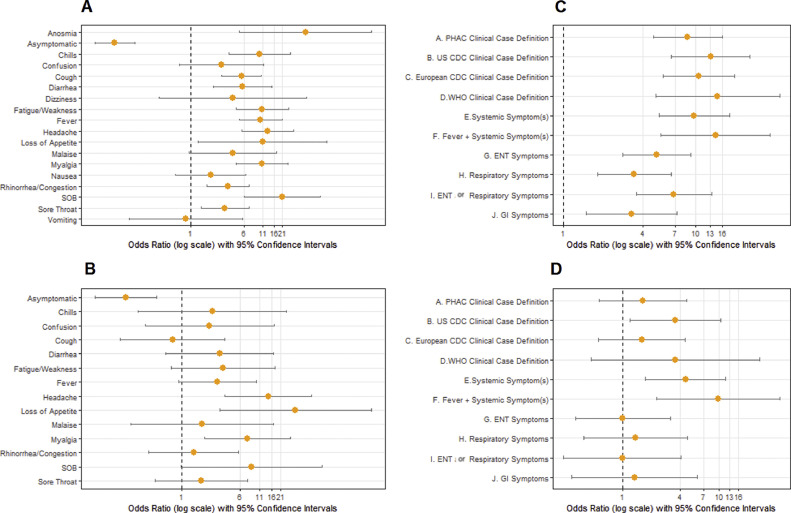
Fig 1.
Odds of serological reactivity based on symptoms and symptom clusters. (A and B) Depict (age, gender and facility) adjusted odds ratios (aORs) on a log10 scale for seropositivity of individual symptoms among the entire population (1A) or among individuals with a negative or no NAAT test prior to serological testing (1B). Anosmia, dizziness, nausea, and vomiting are not reported in 1B due to extremely broad confidence intervals. (C and D) Depict aORs (on a log10 scale) for seropositivity of symptom clusters (Appendix A) among the entire population (1C) or among individuals with a negative or no NAAT test prior to serological testing(1D). Anosmia, loss of smell/taste; SOB, shortness of breath/difficulty breathing; PHAC, Public Health Agency of Canada; US CDC, United States Centre for Disease Control; European CDC, European Centre for Disease Prevention and Control; WHO, World Health Organization.