Abstract
Intellectual developmental disorder with abnormal behavior, microcephaly and short stature (IDDABS), (OMIM# 618342) is an autosomal recessive condition described as developmental delay, poor or absent speech, intellectual disability, short stature, mild to progressive microcephaly, delayed psychomotor development, hyperactivity, seizure, along with mild to swear aggressive behavior. Homozygous frameshift mutation in Pseudouridine Synthase 7, Putative; (PUS7) OMIM# 616,261 NM_019042.3 and splice acceptor variants in Alpha-Aminoadipic Semialdehyde Synthase; (AASS) OMIM# 605,113 NM_005763.3 was funded. Whole exome sequencing (WES) technique was used as tool to identify the molecular diagnostic test. Different bioinformatics analysis done for WES data and we identified two novel mutations one as frameshift mutation c.606_607delGA, p.Ser282CysfsTer9 in the PUS7 gene and splice acceptor variants c.1767–1 G > A in the AASS gene has been reported. The pattern of family segregation maintained the pathogenicity of this variation associated with abnormal behavior, intellectual developmental disorder, microcephaly along with short stature IDDABS. Further, the WES data was validated in the family having other affected individuals and healthy controls (n = 100) was done using Sanger sequencing. Finally, our results further explained the role of WES in the disease diagnosis and elucidated that the mutation in PUS7 and AASS genes may lead an important role for the development of IDDABS in Saudi family.
Keywords: IDDABS, Intellectual developmental disorder, PUS7, AASS, Dysmorphic facies, WES, Saudi family
1. Introduction
Intellectual developmental disorder with abnormal behavior, microcephaly and short stature (IDDABS) is an autosomal recessive disorder is caused by homozygous mutation in the PUS7 gene (616261) that is located on chromosome 7q22. De Brouwer et al., 2018, Shaheen et al., 2019, reported five unrelated consanguineous families from Pakistan, Egypt, Syrian, Moroccan and Saudi descent with nine patients ranged in age from 2 to 18 years old with developmental delay and impaired intellectual development (ID). They all had poor overall growth with short stature, low weight, and microcephaly and some patients had normal motor development, whereas others had delayed walking. The patients had moderate intellectual disability, poor or absent speech, and behavioral abnormalities, including aggression and short temper with variable dysmorphic features, including smooth philtrum, epicanthal folds, down slanting palpebral fissures, broad nasal root, full lips, everted lower lip, hypodontia, and misaligned or conical shaped teeth. All the patients showed different homozygous and frameshift mutations in the PUS7 gene (De Brouwer et al., 2018, Shaheen et al., 2019). Patient-derived cells showed decreased pseudouridylation signals specifically for PUS7 substrates at position 13 of at least 10 different cytosolic tRNAs, but not at other positions. Pseudouridylation of mRNA was also decreased, but the functional significance of this observation was unclear. The findings indicated that RNA pseudouridylation, along with tRNA modification is important for proper neural development (De Brouwer et al., 2018).
Studies in pus7 mutant yeast showed that the D478Y mutant (equivalent to D503Y in humans) was unable to complement a growth defect, indicating that this missense mutation has a loss-of-function effect (Shaheen et al., 2019).
PUS7 gene encodes the RNA-independent pseudouridylate synthase 7. Pseudouridylation is the most abundant post transcriptional modification in RNA, which is primarily thought to stabilize secondary structures of RNA (Charette and Gray, 2000, Zebarjadian et al., 1999). Pseudouridine (5-ribosyluracil, ) is the 5-ribosyl isomer of uridine. Pseudouridylation in human is catalyzed by 13 pseudouridine synthases (PUSs), which are either guided to their targets via small nucleolar RNAs (snoRNAs) or directly recognize their targets in an RNA-independent manner. (Charette and Gray, 2000, Spenkuch et al., 2014).
PUS7 gene is known that it translates RNA-independent pseudouridylate synthase 7. It has been explained previously that in yeast and human PUS7 identify a core motif UG AG. (Schwartz et al., 2014, Safra et al., 2017). In the Yeast at position 35 PUS7 modifies U2 snRNA, (Yu et al., 2011, Schwartz et al., 2014) reported modifications of numerous tRNAs at position 13 and position 35 in pre-tRNA(Tyr) (Behm-Ansmant et al., 2003), and further modifies a abundance of mRNAs, in specific upon a heat shock. Moreover, the PUS7 in human was known to transform tRNAs and tRNA-derived small fragments (tRFs) (Guzzi et al., 2018). Pseudouridylation of tRFs is very important step that is obligatory for early embryogenesis and for hematopoiesis. Furthermore, human PUS7 gene play an important role to targets hundreds of different mRNAs associated in a range of functions (Schwartz et al., 2014, Safra et al., 2017, Li et al., 2015)
Our WES study also identified a novel splice acceptor variants c.1767–1 G > A in the Alpha-Aminoadipic Semialdehyde Synthase (AASS) gene in same family. The AASS gene encodes two enzymes lysine alpha-ketoglutarate reductase (LKR) along with saccharopine dehydrogenase (SDH) are recognized to play a significant role for synthesis of alpha-aminoadipic semialdehyde synthase. The lysine degradation pathway this bifunctional protein translate by AASS gene involved in the first 2 steps of catalyzes. These steps of enzyme activities involved in the adaptation of lysine to alpha-aminoadipic semialdehyde (Sacksteder et al., 2000, Tondo et al., 2013).
Patient with hyperlysinemia Type 1 (OMIM# 238700) having nonspecific seizure, hypotonia, mildly delayed psychomotor development, while patients with Saccharopinuria (OMIM# 268700) show mild developmental delay, hyperactivity and speech delay. Dancis et al., 1976, Sacksteder et al., 2000 reported homozygosity of 9-bp deletion in the AASS gene at 15th exon in a consanguineous family resulting a early stop codon at 534 location of the protein leading to hyperlysinemia type I. In 2 unrelated patients with hyperlysinemia type I, infantile seizures, and mild cognitive deficits, Tondo et al., 2013 reported a compound heterozygous splice site mutations in the AASS gene in an Argentinian girl that was leading to a deletion/insertion mutation (c.2662 + 1_2662 + 5delGTAAGinsTT) resulting as a frameshift, and premature termination. While in other patient a c.874A-G transition in intron 8 (605113.0003), resulting in a heterozygous in-frame deletion of the 21 bp in exon 8 (c.874_894del) A loss of function occurs due to splicing mutations leading to resulted in truncated proteins reported by (Tondo et al., 2013). Further, a Spanish 8 years old boy with hyperlysinemia type I, reported having a compound heterozygous mutations in the AASS gene again there was a 2-bp deletion (c.976delCA), result in a frameshift and premature termination (Gln326fs), and a c.1925C > G transversion, resulting in a ser642-to-ter (S642X) replacement (Tondo et al., 2013). Moreover, another study on 3 patients, including 2 sibs, with variable neurologic deficits and hyperlysinemia type I) reported a c.194G-A mutation in AASS gene, resulting a replacement of highly conserved residue in an arg65-to-gln (R65Q) in exon 2. One patient was homozygous for the mutation, whereas the 2 sibs were compound heterozygous for R65Q and a c.1256 T-G transversion in exon 11, resulting in a leu419-to-arg (L419R) (Houten et al., 2013).
In this study, we described a consanguineous Saudi family having two mutations one in PUS7 and other in AASS genes for the first time and both variants are segregating a different like homozygous variant in PUS7 as frameshift deletion and splice acceptor variants in AASS gene. The related phenotype is consistent and includes intellectual disability seizure, hypotonia, short stature and progressive microcephaly. Our findings further expand the possible role of WES for diagnosis of disease and explained that the mutation in PUS7 and AASS genes play an important role for the development of IDDABS in Saudi family.
2. Materials and methods
2.1. Ethical approval and samples collection
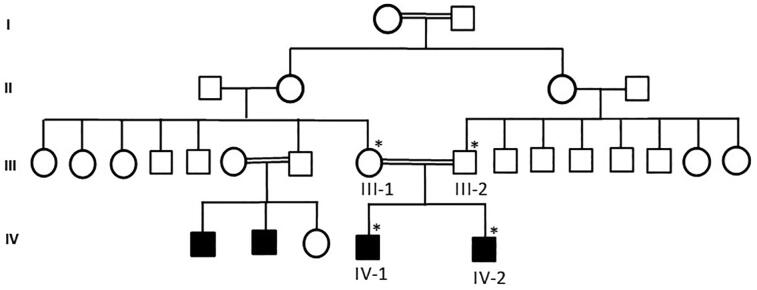
The recruitment of the family for blood samples was done according to the ethical protocols and established guidelines. Blood samples were taken after signing the consent from all the participants following the protocol according to the Declaration of Helsinki. Ethical approval for this study was taken from the ethical committee of the CEGMR, KAU. Peripheral blood samples was used to extract the DNA using the MegNA Pure 96 kit using the protocol as mentioned (Roche Life Science). Family pedigree was drawn after having the information from the parents as shown in the Fig. 1. The DNA concentration was checked by using NanodropTM 2000/2000c spectrophotometers Thermo Fisher Scientific. Samples for WES was prepared using the Agilent Sure Select Target Enrichment Kit. Samples were collected from all family members and 100 healthy samples from Saudi Arabia. Family pedigree was drawn as shown in Fig. 1.
Fig. 1.
A detailed family pedigree was drawn after having the detailed information from the parents. The available samples were marked as symbol satiric.
2.2. Clinical report of the patient
Patient (IV-1) is 4 years old boy was born by cesarean section delivery at full term without any complication. Newborn underwent for the oxygen supply immediately after delivery as was feeling problem in breathing. He has one brother with abnormal growth conditions. The proband has dysmorphic features, developmental delay and intellectual disability. He has hearing loss can’t speak. He was diagnosed as microcephaly with head circumference of 39.4 (-2.8SD), dysmorphic features and brain atrophy. He started walking with support at the age of 2 years. He had ID, gross motor delay along with hypotonia. He was also reported difficulties in swallowing with nonspecific seizure, hypotonia, mildly delayed psychomotor development.
Patient (IV-2) is 7 years old boy was born by cesarean section delivery at full term having no complication. The proband has dysmorphic features, speech delay developmental delay and intellectual disability. He has hearing loss can’t speak. At the age of one and half years he started walking without support. He also had hypotonia, gross motor delay along with intellectual disability.
2.3. Whole exome sequencing
To do the WES DNA of the affected individual was enriched for the whole coding region and splice site junctions of all the genes. The products are sequenced on an illumine NextSeq instrument with 2x76 paired end reads as previously done (Naseer et al., 2020). The sequence is compared to a reference sequences GRCH37/UCSC hg19 human genome build. All sequence alterations are defined by using the Human Genome Variation society nomenclature guidelines. Various bioinformatics analyses were carried to identify causative variant in PUS7 and AASS genes. Variants obtained from sequencing were filtered for novel and rare (MAF = 0.01%), functional (predicted damaging by SIFT/Polyphen), the heterozygous/homozygous state in the affected individual literature search and database queries were also performed. WES data were mapped against the information in the dbSNP (http://www.Ncbi.Nlm.Nih.Gov/snp/), and population based data base such as genome aggregation database genomAD, Exome Variant Servise (ESP) and 1000 Genomes databases (http://www.1000genomes.Org/data) and disease based database such as ClinVar (Database of assertions about the clinical significance and phenotype relationship of human variations), OMIM (Database of human genes and genetic conditions that also contains a representative sampling of disease-associated genetic variants) and Human Gene Mutation (Database of variant annotations published in the literature). Following by the standard guideline of ACMG.
In silico functional study was done for the current mutations to check deleterious effect and abnormalities caused by mutations. For the in silico predictions we used software’s like Mutation Tester (http://www.mutationtaster.org/), 1000 Genomes (http://www.internationalgenome.org/), PhyloP (https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC4702902/), PhyloP
GERP++ (http://mendel.stanford.edu/SidowLab/ downloads/gerp/), SIFT
(http://sift.bii.a-star.edu.sg/), PhastCons (http://compgen.cshl.edu/phast/), CADD (https://cadd.gs.washington.edu/), SiPhy (https://omictools.com/siphy-tool) and Exome Aggregation Consortium (http://exac.broadinstitute.org/), PROVEAN and MAPP for Protein structure/function and evolutionary conservation. All the software predict this variant as disease causing.
2.4. Sanger sequencing
Mutations identified using WES were further validated by Sanger sequencing analysis. Data files of sequencing were obtained from the AB1 sequencing unit. Sequencing file was aligned with reference sequence using the BioEdit software. We followed guidelines to search for the variations in the National Center for Biotechnology Information SNP database. Further, to rule out this mutation in normal population 100 control samples were also sequenced for these identified variants.
3. Results
3.1. Whole Exome sequencing
After the WES the variant call format (vcf) file having total of 101,755 variants were identified. Bioinformatics analysis were used to find out the causative variant by applying different filters based on the pathogenicity, genomic position, frequency, quality, protein effect and having any relations with the diseased phenotype. Further the variants obtained from WES were filtered to include only novel/rare (MAF = 0.01%), present in the heterozygous or homozygous state predicted damaging by Polyphen/SIFT software. We detected two mutations one homozygous frameshift mutation c.606_607delGA, p.Ser282CysfsTer9 in the PUS7 gene and other splice acceptor variants c.1767–1 G > A in the AASS gene in the analysis. These variant are absent in gnomAD and dbSNP database. Moreover, multiple in silico tools also used and they predict a harmful effect of these mutations as shown in Table 1.
Table 1.
Showing the details of in silico analysis done for this study.
| S. No | Online Tools | Pathogenicity Score for mutation in PUS7 gene c.606_607delGA |
Pathogenicity Score for mutation AASS position gene c.1767–1 G > A |
|---|---|---|---|
| 1 | SIFT | 0.01 | 0.01 |
| 2 | Exome Aggregation Consortium Version 0.3.1 |
0.0 | 0.0 |
| 3 | 1000 Genomes | 0.0 | 0.0 |
| 4 | Polyphen-2 (v2.2.2, released in Feb 2013) |
0.72 | 0.82 |
| 5 | MutationTaster | Disease causing | Disease causing |
| 6 | MutationAssessor 2.0 | 1.23 | 2.22 |
| 7 | Phastcons 1.4 | 1.0 | 1.0 |
| 8 | PhyloP (phyloP46way_placental) | 0.98 | 1.06 |
| 9 | SiPhy 0.5 | 17.07 | 19.27 |
3.2. Sanger sequencing analysis
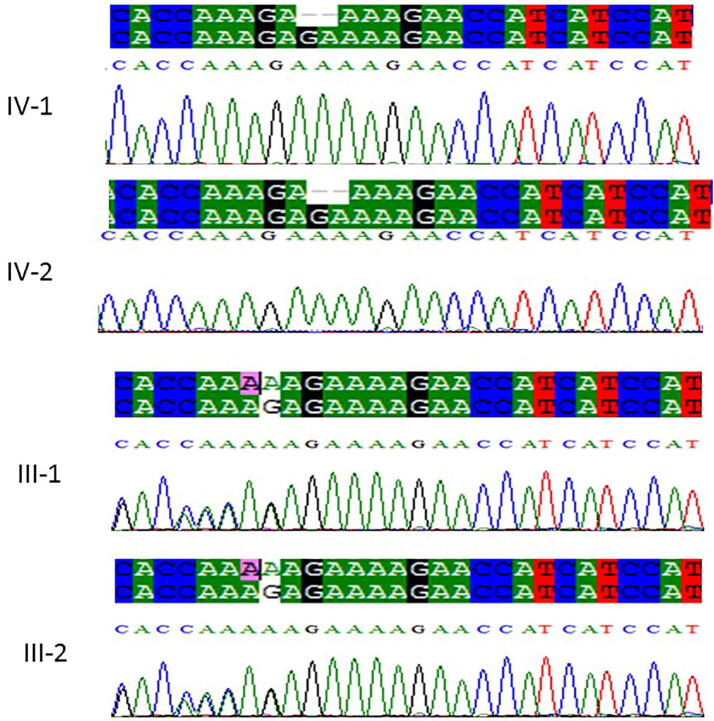
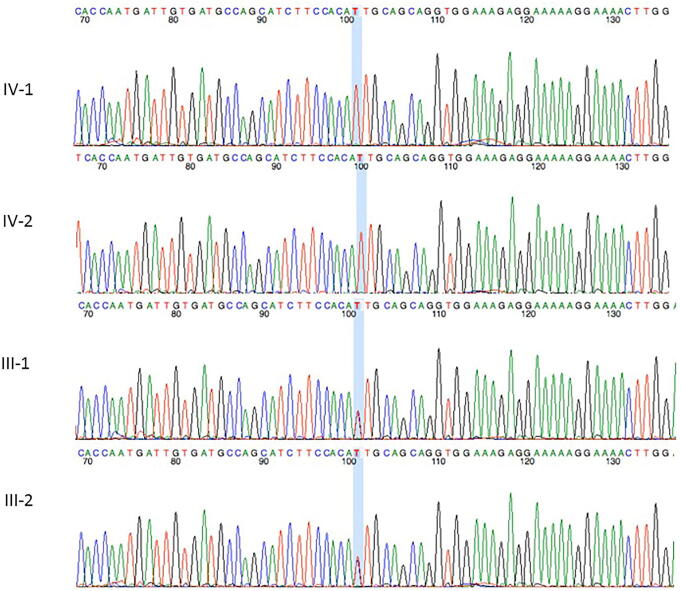
Sanger sequencing technique was used to validate the WES sequencing results by designing the primers that were designed for the observed mutation. The results of Sanger sequencing showed homozygous frameshift mutation c.606_607delGA, p.Ser282CysfsTer9 in the PUS7 as shown in Fig. 2. Moreover, Sanger sequencing also confirmed a splice acceptor variants c.1767–1 G > A in the AASS gene as shown in Fig. 3. This resulted mutations were ruled out in 100 healthy controls samples from the population.
Fig. 2.
Chromatogram of Sanger sequence analysis III-1 and III-2 are the normal parents, while (IV-1 and IV-2) are the affected member of the family showing a novel frameshift mutation c.606_607delGA, p.Ser282CysfsTer9 in the PUS7 gene.
Fig. 3.
Sanger sequencing analysis showed a novel splice acceptor variants c.1767–1 G > A in the AASS gene in the affected member of the family.
3.3. Gene interactions and pathways
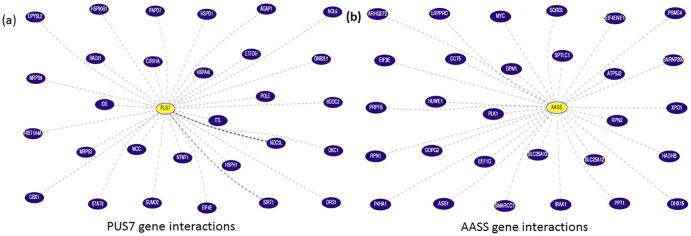
Gene interactions along with pathways from curated databases and text mining was done to find out the relations of identified genes with other top 30 genes. UCSC gene browser was used to find out the possible interactions of PUS7 and AASS gene with other genes as shown in Fig. 4(a,b). Moreover, the intensive database search and literature review was done to limit the variants to genes relevant to the clinical history.
Fig. 4.
PUS7 and AASS genes pathways was drawn from databases and text-mining according to USCS gene browser.
4. Discussion
A large number of studies have been done to explain tRNA modification in different human diseases that are linked Mendelian disorders (Ramos and Fu, 2018, Torres et al., 2014). In spite of the plenty of these post translation modifications of tRNA, its biological significance has been limited to the phenotypes in human that is associated with PUS1 and PUS3 mutations (Fernandez-Vizarra et al., 2007, Shaheen et al., 2016).
In previous studies it has reported that mutations in PUS1 and PUS3 (MIM #600462, 616283) genes respectively leading to speech and motor impairments along with ID syndromes (Bykhovskaya et al., 2004, Shaheen et al., 2016). Further, current identification of three unrelated families with ID showed pathogenic PUS7 c.382G > A (p.Gly128Arg) mutations reinforced the possibility of as the disease causing mutation (De Brouwer et al., 2018). Moreover, this pathogenic PUS7 mutation was segregated with disease and result of change an evolutionary conserved glycine residue. Further the mutation of p.Gly28Arg in PUS7 present in the TruD domain of Pseudouridine synthase suggest that this change disrupt pseudouridylation as described. Darvish et al., 2019 gave father evidence by providing the clinical and genetic information of family reported with ID, motor impairments, speech delay, and mild to aggressive behavior as a result of pathogenic mutation in PUS7 gene and these changes are associated with pseudouridylation. Furthermore, the two other families having the point mutation in a highly conserved residue of PUS7 that is involved in a salt bridge in the E. coli TruD homolog (Kaya et al. 2004).
Furthermore, any damaging effect or change during protein synthesis due to the mutations in tRNA modification genes in central nervous system may lead to the Mendelian diseases (Kirchner and Ignatova, 2015, Ramos and Fu, 2018, Shaheen et al., 2015, Torres et al., 2014). Recently, a number of studies explain the promising role of the protein translation in many brain disorders that increased the list of the number of diseases due to mutation in different component of protein translation and its modifications (Kapur and Ackerman, 2018, Tahmasebi et al., 2018). Although the advantage of discovering these link of the gene with disease as described in this study, is currently limited to build an accurate molecular diagnosis and prevention through informed reproductive choices, it is likely that these revelations will inform the development of therapeutics in the future. The associated consistent defect in pseudouridylation provides hints at the potential pathogenesis of this syndrome. In addition, this may also serve as a very helpful assay for the proper classification of variants of unknown significance that will inevitably be encountered in this gene as exome/genome sequencing of children with intellectual disability will incorporate it in their annotation. The presence of similar phenotypes such as ID, short stature or microcephaly in this family reflect genotype phenotype correlation, since this family presented with a homozygous frameshift mutation in PUS7 gene similar as previously reported families carried nonsense or frameshift mutations that may cause loss of function. This is the first report of a frameshift mutation in PUS7 gene as a pathogenic gene for ID syndromes with speech impairments and aggressive behaviors in Saudi population. Finally, it is established that in humans PUS7 gene mutations produce a syndrome of ID, progressive microcephaly delayed psychomotor development, hyperactivity, along with mild to swear aggressive behavior and many other variable features.
Moreover, the role of AASS gene is also very important. In the animal model study of C. elegans it was observed that mutations affecting only the SDH domain of Aass1, of AASS gene, resulting the abnormal enlargement of mitochondrial in hypodermis (Zhou et al. 2019). In C. elegans animal model the mutations disturbing the LKR domain only or both the SDH and LKR domains that resulted in lysine accumulation, whereas mutations in the SDH domain only resulted in saccharopine accumulation. Expression of human AASS in mutant C. elegans rescued these phenotypes, indicating evolutionarily conserved function between C. elegans and mammals. Furthermore, these defects may lead to growth abnormailties at postnatal stages and death of Aass SDH mutant mice (Zhou et al., 2019). Mostly, the amino acid catabolism take place in mitochondria, so any anomalous amino acid catabolism may result in defect of mitochondria and functional loss. Any mutation may lead to mitochondrial homeostasis related to metabolic stresses and leads to the aminoacidopathies of unknown etiology.
5. Conclusions
In conclusion in this study we identified a family having two novel mutations one as frameshift mutation c.606_607delGA, p.Ser282CysfsTer9 in the PUS7 gene and splice acceptor variants c.1767–1 G > A in the AASS gene. The pattern of family segregations support the pathogenicity of this variant associated with abnormal behavior, intellectual developmental disorder, microcephaly along with short stature leading to IDDABS. Finally, our results further explained the role of WES in the efficient disease diagnosis and elucidated that the mutations in PUS7 and AASS genes may play a significant role for the development of IDDABS for the first time in Saudi family.
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. (DF-493-142-1441). The authors, therefore, gratefully acknowledge DSR technology and financial support.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Muhammad Imran Naseer, Email: minaseer@kau.edu.sa.
Angham Abdulrahman Abdulkareem, Email: aabdulkareem@kau.edu.sa.
Mohammed M. Jan, Email: mmjan@kau.edu.sa.
Adeel G. Chaudhary, Email: chaudhary@kau.edu.sa.
Shatha Alharazy, Email: s.alharazy@surry.ac.ik.
Mohammad H. AlQahtani, Email: mhalqahtani@kau.edu.sa.
References
- De Brouwer A.P.M., Abou J.R., Kortel N. Variants in PUS7 cause intellectual disability with speech delay, microcephaly, short stature, and aggressive behavior. Am J Hum Genet. 2018;103:1045–1052. doi: 10.1016/j.ajhg.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R., Tasak M., Maddirevula S., Abdel-Salam G.M.H., Sayed I.S.M., Alazami A.M., Al-Sheddi T., Alobeid E., Phizicky E.M., Alkuraya F.S. PUS7 mutations impair pseudouridylation in humans and cause intellectual disability and microcephaly. Hum. Genet. 2019;138:231–239. doi: 10.1007/s00439-019-01980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charette M., Gray M.W. Pseudouridine in RNA: what, where, how, and why. IUBMB Life. 2000;49:341–351. doi: 10.1080/152165400410182. [DOI] [PubMed] [Google Scholar]
- Zebarjadian Y., King T., Fournier M.J., Clarke L., Carbon J. Point mutations in yeast CBF5 can abolish in vivo pseudouridylation of rRNA. Mol. Cell. Biol. 1999;19:7461–7472. doi: 10.1128/mcb.19.11.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spenkuch F., Motorin Y., Helm M. Pseudouridine: still mysterious, but never a fake (uridine)! RNA Biol. 2014;11:1540–1554. doi: 10.4161/15476286.2014.992278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Bernstein D.A., Mumbach M.R., Jovanovic M., Herbst R.H., León-Ricardo B.X., Engreitz J.M., Guttman M., Satija R., Lander E.S. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell. 2014;159:148–162. doi: 10.1016/j.cell.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safra M., Nir R., Farouq D., Vainberg Slutskin I., Schwartz S. TRUB1 is the predominant pseudouridine synthase acting on mammalian mRNA via a predictable and conserved code. Genome Res. 2017;27:393–406. doi: 10.1101/gr.207613.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A.T., Ge J., Yu Y.T. Pseudouridines in spliceosomal snRNAs. Protein. Cell. 2011;2:712–725. doi: 10.1007/s13238-011-1087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm-Ansmant I., Urban A., Ma X., Yu Y.T., Motorin Y., Branlant C. The Saccharomyces cerevisiae U2 snRNA:pseudouridine-synthase Pus7p is a novel multisitemultisubstrate RNA:Psi-synthase also acting on tRNAs. RNA. 2003;9:1371–1382. doi: 10.1261/rna.5520403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzi N., Ciesla M., Ngoc P.C.T., Lang S., Arora S., Dimitriou M., Pimkova K., Sommarin M.N.E., Munita R., Lubas M. Pseudouridylation of tRNA-derived fragments steers translational control in stem cells. Cell. 2018;173(1204–1216) doi: 10.1016/j.cell.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Li X., Zhu P., Ma S., Song J., Bai J., Sun F., Yi C. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat. Chem. Biol. 2015;11:592–597. doi: 10.1038/nchembio.1836. [DOI] [PubMed] [Google Scholar]
- Dancis J., Hutzler J., Woody N.C., Cox R.P. Multiple enzyme defects in familial hyperlysinemia. Pediat. Res. 1976;10:686–691. doi: 10.1203/00006450-197607000-00011. [DOI] [PubMed] [Google Scholar]
- Sacksteder K.A., Biery B.J., Morrell J.C., Goodman B.K., Geisbrecht B.V., Cox R.P., Gould S.J., Geraghty M.T. Identification of the a-Aminoadipic Semialdehyde Synthase Gene, Which Is Defective in Familial Hyperlysinemia. Am. J. Hum. Genet. 2000;66:1736–1743. doi: 10.1086/302919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondo M., Calpena E., Arriola G., Sanz P., Martorell L., Ormazabal A., Castejon E., Palacin M., Ugarte M., Espinos C., Perez B., Perez-Dueñas B., Pérez-Cerda C., Artuch R. Clinical, biochemical, molecular and therapeutic aspects of 2 new cases of 2-aminoadipic semialdehyde synthase deficiency Molecular Genetics and. Metabolism. 2013;110:231–236. doi: 10.1016/j.ymgme.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Houten S.M., Brinke H., Denis S., Ruiter J.P.N., Knegt A.C., de Klerk J.B.C., Augoustides-Savvopoulou P., Haberle J., Baumgartner M.R., Coskun T., Zschocke J., Sass J.O., Poll-The B.T., Wanders R.J.A., Duran M. Genetic basis of hyperlysinemia. Orphanet J. Rare Dis. 2013;8:57. doi: 10.1186/1750-1172-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseer M.I., Abdulkareem A.A., Guzmán-Vega F.J., Arold S.T., Pushparaj P.N., Chaudhary A.G., AlQahtani M.H. Novel Missense Variant in Heterozygous State in the BRPF1 Gene Leading to Intellectual Developmental Disorder With Dysmorphic Facies and Ptosis. Front Genet. 2020;7(11):368. doi: 10.3389/fgene.2020.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J., Fu D. The emerging impact of tRNA modifications in the brain and nervous system. Biochim Biophys Acta Gene Regul Mech. 2018;1862(3):412–428. doi: 10.1016/j.bbagrm.2018.11.007. [DOI] [PubMed] [Google Scholar]
- Torres A.G., Batlle E., de Pouplana L.R. Role of tRNA modifications in human diseases. Trends Mol. Med. 2014;20:306–314. doi: 10.1016/j.molmed.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Fernandez-Vizarra E., Berardinelli A., Valente L., Tiranti V., Zeviani M. Nonsense mutation in pseudouridylate synthase 1 (PUS1) in two brothers affected by myopathy, lactic acidosis and sideroblastic anaemia (MLASA) J Med Genet. 2007;44:173–180. doi: 10.1136/jmg.2006.045252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R., Han L., Faqeih E. A homozygous truncating mutation in PUS3 expands the role of tRNA modification in normal cognition. Hum Genet. 2016;135:707–713. doi: 10.1007/s00439-016-1665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykhovskaya Y., Casas K., Mengesha E., Inbal A., Fischel-Ghodsian N. Missense mutation in pseudouridine synthase 1 (PUS1) causes mitochondrial myopathy and sideroblastic anemia (MLASA) Am J Hum Genet. 2004;74:1303–1308. doi: 10.1086/421530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvish H., Azcona L.J., Alehabib E., Jamali F., Tafakhori A., Ranji-Burachaloo S., Jen J.C., Paiśan-Ruiz C. A novel PUS7 mutation causes intellectual disability with autistic and aggressive behaviors. Neurol Genet. 2019;5:e356. doi: 10.1212/NXG.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya Y., Del Campo M., Ofengand J., Malhotra A. Crystal structure of TruD, a novel pseudouridine synthase with a new protein fold. J. Biol. Chem. 2004;279:18107–18110. doi: 10.1074/jbc.C400072200. [DOI] [PubMed] [Google Scholar]
- Kirchner S., Ignatova Z. Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nat. Rev. Genet. 2015;16:98. doi: 10.1038/nrg3861. [DOI] [PubMed] [Google Scholar]
- Shaheen R., Abdel-Salam G.M., Guy M.P., Alomar R., Abdel-Hamid M.S., Afifi H.H., Ismail S.I., Emam B.A., Phizicky E.M., Alkuraya F.S. Mutation in WDR4 impairs tRNA m 7 G 46 methylation and causes a distinct form of microcephalic primordial dwarfism. Genome Biol. 2015;16:210. doi: 10.1186/s13059-015-0779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebi S., Khoutorsky A., Mathews M.B., Sonenberg N. Translation deregulation in human disease. Nat Rev. Mol. Cell Biol. 2018;19:791–807. doi: 10.1038/s41580-018-0034-x. [DOI] [PubMed] [Google Scholar]
- Kapur M., Ackerman S.L. mRNA Translation Gone Awry: Translation Fidelity and Neurological Disease. Trends Genet. 2018;34(3):218–231. doi: 10.1016/j.tig.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Wang X., Wang M., Chang Y., Zhang F., Ban Z., Tang R., Gan Q., Wu S., Guo1 Y., Zhang Q., Wang F., Zhao L., Jing Y., Qian W., Wang G., Guo W., Yang C. The lysine catabolite saccharopine impairs development by disrupting mitochondrial homeostasis. J. Cell Biol. 2019;218(2):580–597. doi: 10.1083/jcb.201807204. [DOI] [PMC free article] [PubMed] [Google Scholar]