Figure 3.
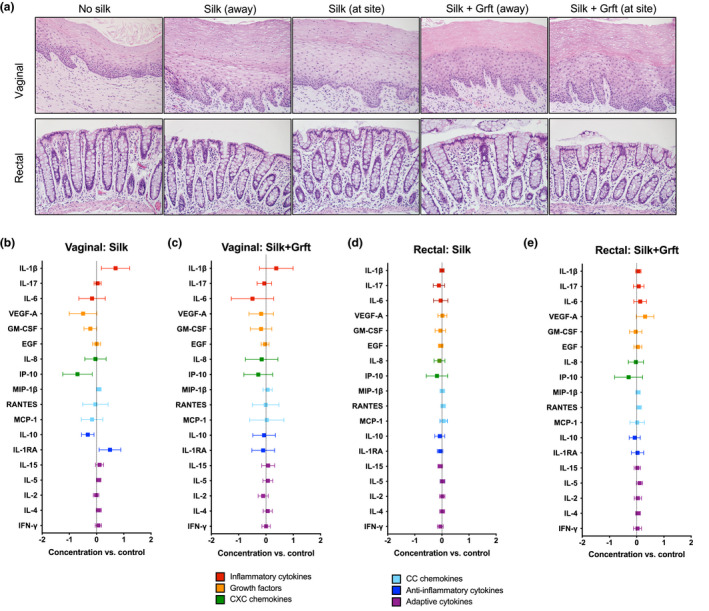
Safety profile of SF‐Grft delivery in vaginal and rectal compartments. (A) H&E stains were obtained from vaginal and rectal tissue biopsies in the following groups: (1) control, (2) following exposure to SF (Silk) at and away from the placement site, and (3) following exposure to SF‐Grft (Silk + Grft) at and away from the placement site. Inflammatory signatures were detected by a multi‐plex Luminex assay in culture supernatant of vaginal explants following (B) in vivo exposure to SF or (C) SF‐Grft and rectal explants following (D) in vivo exposure to SF or (E) SF‐Grft. All were compared to the culture supernatant of tissue explants from control animals.
