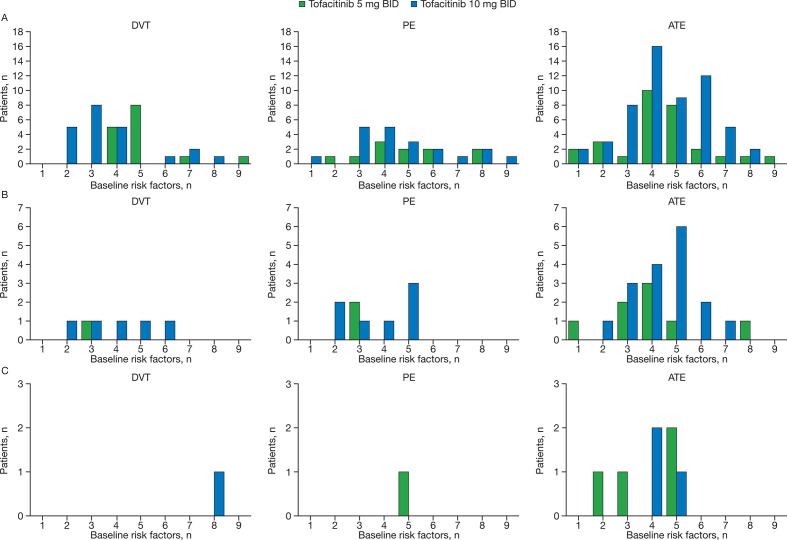
Figure 5.
Number of baseline risk factors for patients who experienced a DVT, PE or ATE in completed studies in the (A) RA, (B) PsO and (C) PsA tofacitinib development programmes, stratified by tofacitinib dose. Those patients who had an event within the predefined risk period (minimum of a patient’s last treatment dose date plus 28 days, date of death or up to the last observation date) are included. Details on the individual risk factors, and the number of patients who experienced each risk factor, are summarised in online supplementary table S6. ATE, arterial thromboembolism; BID, twice daily; DVT, deep vein thrombosis; n, number of patients with a given number of baseline risk factors; PE, pulmonary embolism; PsA, psoriatic arthritis; PsO, psoriasis; RA, rheumatoid arthritis.