Abstract
Background:
Serum Amyloid A (SAA) is an acute phase protein and we analyzed its concentrations in lung transplantated patients (LTX).
Methods:
26 LTX patients (58.6 ± 11 years) and 11 healthy controls (55 ± 11.3 years). Three groups of LTX patients: acute rejection (AR, 7) bronchiolitis obliterans syndrome (BOS, 3), acute infection (INF, 9) and stable patients (NEG, 7).
Results:
In LTX patients SAA concentrations were significantly increased, particularly in AR and INF. In LTX-AR patients were observed a correlation between SAA levels and peripheral CD4+ lymphocyte percentage (r=0.9, p<0.01) and a reverse correlation with FVC percentages (r -0.94, p=0.01).
Conclusions:
SAA may represent a potential biomarker of LTX acute complications, with a prognostic value in AR. (Sarcoidosis Vasc Diffuse Lung Dis 2020; 37 (1): 2-7)
Keywords: serum amyloid A, serum biomarkers, lung transplantation
Introduction
Lung transplantation (LTX) is an option for end-stage lung disease patients no longer responsive to optimal medical therapy or for which no effective medical or surgical treatment is available (1,2). Complications of LTX include Acute Rejection (AR), a complex manifestation of vascular and parenchymal damage due to the action of T lymphocytes, macrophages and other inflammatory mediators. AR commonly occurs at least one week after LTX, time necessary for the differentiation of the effector T lymphocytes and for the production of specific antibodies (3). T lymphocytes play a decisive role in AR by responding to alloantigens, including MHC molecules, expressed on endothelial and parenchymal transplant cells. Once activated, T lymphocytes induce direct lysis of transplanted cells and cytokine production facilitating the recruitment and activation of inflammatory cells leading to tissue necrosis (3). The gold standard for AR diagnosis is transbronchial biopsy. There are various degrees of rejection: in most advanced stages, there is also a widespread commitment to alveolar and interstitial space (3). AR is clinically associated with nonspecific signs, consisting of cough, dyspnoea, and fever, associated with a functional reduction of FEV1 and FVC >10%. High-resolution CT scan (HRCT) cannot differentiate this condition from other complications such as reperfusion injury and infections. Chronic lung graft dysfunction (CLAD) constitutes the first cause of death after the first year from the transplant and is characterized by a histological pattern of obliterative bronchiolitis (BOS) (4). BOS is a syndrome caused by an irreversible obstruction of the distal airways associated with progressive dyspnea and coughing; HRCT can reveal hyperinsufflation and bronchiectasis. The initial pathological process of BOS is characterized by a lymphocyte infiltration of the submucosa and the airway epithelium, a condition known as lymphocytic bronchiolitis (3, 4).
Infections of the respiratory tract are associated with infiltrates at chest X-ray scan and with reduction of pulmonary function. For this reason, it is commonly difficult to differentiate, in LTX patients, infections from rejection on the basis of clinical and radiological evaluations (5, 8) and it is generally necessary to perform bronchoscopy with BAL and transbronchial biopsy. After the first month of transplantation, in fact the reactivation of latent infections from viruses (Herpes and Cytomegalovirus), or fungi (such as Pneumocystis Jirovecii) is a common event that is necessary to recognize and differentiate from AR (8, 9).
Serum amyloid A (SAA) is an acute phase protein with multiple immunological functions involved in lipid metabolism and inflammatory reactions. Four genes on chromosome 11 responsible for the synthesis of SAA1, SAA2, SAA3 and SAA4 have been identified in the human genome (10, 11). A-SAA is a single polypeptide of 104 amino acid residues; although its exact tertiary structure is unknown, the critical role of the 10 amino-terminal residues is recognized in the formation of amyloid fibrils that bind high density lipoprotein (HDL) (10). Proteins SAA1 and SAA2 act as acute phase reactants in plasma and are also plasma precursors of amyloid A fibrils (12-15). SAA has been studied in many biological fluids from patients with different lung diseases, including lung cancer and obstructive/restrictive lung diseases (10,12). Most plasma A-SAA is synthesized by the liver, although extrahepatic synthesis by macrophages, endothelial cells, smooth muscle fibrocells, adipocytes and cancer cells has been described (13, 15). Its role as a potential prognostic biomarker has been suggested in carcinogenesis process (16), granulomatous interstitial lung diseases (17) and obstructive lung disorders (18-20).
This pleiotropic protein with various immunological functions has never been evaluated in patients undergoing LTX: this study aims to evaluate SAA behavior in different conditions of LTX patients and its prognostic value.
Materials and Methods
Study population and design of the study
SAA levels were evaluated in a population of 26 patients undergoing LTX at Siena Regional Referral Center for Sarcoidosis and other Interstitial Lung Diseases, Lung Transplantation Unit. The indication and timing of the LTX were set according to international guidelines (25). These 26 patients (13 males, 58.6 ± 11 years) included: 16 single and 10 bilateral transplants. As a control group, SAA were measured in a population of 11 healthy controls (3 males, 55 ± 11.3 years). LTX group was affected by: Idiopathic Pulmonary Fibrosis (IPF) (n = 12), Cystic Fibrosis (n = 5), Bronchiectasis (n = 1), Pulmonary emphysema (n = 5). Patients undergoing LTX were further subdivided, based on clinical, radiological and histological data, in different groups: AR (7 patients, 3 males, 51.4 ± 5.9 years); BOS (3 patients, 2 males, 64.6 ± 5.7 years); infection (INF) (9 patients, 7 males, 60.1 ± 7.5 years); Stable patients (NEG; 7 patients, 3 males, 48.4 ± 16.1 years). Clinical, functional and radiological data were collected from all these patients in a specific database. In particular, the following parameters were taken into consideration:
- Serum evaluations: blood count, ESR, CRP, PT%, APTT sec, fibrinogen, D-dimer, LDH, creatinine, urea, total bilirubin, direct bilirubin, GOT, GPT, ferritin, transferrin, C3 - C4, IgA, IgG, IgM, total cholesterol, cholesterol HDL, LDL cholesterol, triglycerides, albumin (% and absolute value of the predicted), alpha1- alpha2-β1-β2-γ globulins (%).
- Lymphocytic typing of peripheral venous blood: Cell differential count was performed on cytocentrifuge preparations. Peripheral lymphocyte phenotype was characterized using flow cytometry (FacsCantoII; Becton&Dickinson) and monoclonal antibodies (anti-CD3, CD4, CD8, CD19, NK and CD4/CD8; Becton&Dickinson).
- Bronchoalveolar lavage (BAL): Bronchoscopy with BAL was performed in all patients for diagnostic reasons. Bronchoalveolar lavage cell population were reported as percentages of macrophages, lymphocytes, neutrophils and eosinophils. Lymphocyte phenotype was analyzed by flow cytometry (FacsCantoII, Becton Dickinson) using anti -CD3, -CD4, -CD8 monoclonal antibodies.
- Pulmonary function tests (PFR): The following lung function measurements were recorded, using a Jaeger Body Plethysmograph with corrections for temperature and barometric pressure: forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC, total lung capacity (TLC), residual volume (RV), carbon monoxide lung transfer factor (TLCO) and capacity carbon monoxide lung transfer factor/alveolar volume (TLCO/VA). TLCO measurement could not be collected in 10 patients unable to perform properly single-breath maneuver. PFTs were performed after at least 2 hours from exhaled NO measurements.
SAA assay
Serum sampling was performed in all patients after 10 hours of fasting. The assay of the SAA was carried out according to the enzyme immuno-enzymatic method (Enzyme Linked-Immuno-Sorbent Assay) kit Invitrogen (Invitrogen Corporation). The wells of the microplates were coated with a highly purified and specific human monoclonal antibody SAA. During the first incubation, the standards with known SAA contents, controls and samples were pipetted into the coated wells, followed by the addition of a second biotinylated monoclonal antibody. After washing, the enzyme Streptavidin-peroxidase was added; the latter by binding to the biotinylated antibody, has completed the characteristic sandwich of the ELISA method. After a second incubation and a second washing (necessary to remove the unbound enzyme) a solution was added, in order to be accepted by the bound enzyme to produce color. The intensity of the resulting coloring was considered directly proportional to the concentration of Human SAA present in the samples analyzed.
Statistics
Statistical analysis was conducted with GraphPad Prism v 6.0 software for Windows, using non-parametric tests; the differences between groups of two variables were studied with the Mann-Whitney test, while the variance analysis was conducted with the Kruskal-Wallis test. As regards the correlations between the clinical-functional parameters and the values of the SAA, the Spearman correlation index “r” was used. Kaplan Meier curves with log-rank test for curve comparison were conducted for survival analysis. Differences with p <0.05 were considered significant. All data were expressed as mean ± standard deviation, unless otherwise indicated.
Results
The clinical characteristics of the population were reported in Table 1 revealing that the different populations, included in the study, were sex and age matched. Serum levels of SAA in LTX subgroups were listed in Table 2 and they resulted significantly increased in LTX patients compared to healthy controls (p <0.001) (Fig. 1). LTX patients with AR or INF showed significantly higher SAA levels than healthy controls (p = 0.01) and stable LTX patients (NEG) (p <0.05).
Table 1.
Patient demographics, respiratory function tests (PFR) and bronchoalveolar lavage (BAL) cellular composition in patients of the present study with Idiopathic Pulmonary Fibrosis (IPF) and undergoing Pulmonary Transplantation (LTX AR = transplanted with acute rejection, LTX CLAD = transplanted with chronic rejection, LTX INF = transplanted with acute infection, LTX NEG = stable transplanted, CONTROLS=healthy controls); (* n.d = data not available)
| Data | LTX AR | LTX CLAD | LTX INF | LTX NEG | Controls |
| N° pt | 7 | 3 | 9 | 7 | 11 |
| Age (years) | 51,4 ± 5,9 | 64,6 ± 5,7 | 60,1 ± 7,5 | 48,4 ± 16,1 | 55 ± 11.3 |
| Sex (male) | 3 (42,8%) | 2 (66,6%) | 7 (77,7%) | 3 (42,8%) | 3 (27.2%) |
| PFR | |||||
| FEV1 % pred | 66,6 ± 11,2 | n.d* | 61,7 ± 19,9 | 52,6 ± 21,6 | n.d |
| FVC % pred | 72,0 ± 7,6 | n.d | 71,8 ± 8,7 | 63,4 ± 13,2 | n.d |
| ITGV % pred | 109,8 ± 24,1 | n.d | 125,1 ± 71,7 | 139,7 ± 43,4 | n.d |
| TLC % pred | 92,2 ± 13,0 | n.d | 93,8 ± 42,4 | 106,1 ± 27,0 | n.d |
| RV % pred | 138,6 ± 36,5 | n.d | 147,3 ± 98,5 | 196,0 ± 89,4 | n.d |
| DLCO % pred | 45,8 ± 11,3 | n.d | 62,7 ± 9,2 | 57,9 ± 14,8 | n.d |
| KCO % pred | 71,3 ± 10,0 | n.d | 94,2 ± 14,7 | 80,0 ± 25,0 | n.d |
| BAL | |||||
| Cell tot (106) | 6,5 ± 3,8 | n.d | 6,4 ± 4,9 | 9,5 ± 4,7 | n.d |
| Cell 103/ml | 178 ± 50,3 | n.d | 165,2 ± 56,5 | 337 ± 182,4 | n.d |
| Macrophages % | 61,1 ± 31,8 | n.d | 56,3 ± 30,8 | 54,6 ± 36,0 | n.d |
| Lymphocytes % | 15,6 ± 11,2 | n.d | 24,5 ± 29,9 | 22,3 ± 12,8 | n.d |
| Neutrophils % | 16,5 ± 17,4 | n.d | 18,6 ± 15,3 | 23 ± 28,3 | n.d |
| Eosinophils % | 10 ± 17,3 | n.d | 5,6 ± 9,0 | 0 | n.d |
| RCD4/CD8 | 0,6 ± 0,5 | n.d | 0,4 ± 0,3 | 0,3 ± 0,1 | n.d |
Table 2.
Serum levels of SAA in LTX patients (* = groups of patients in the study where the high levels of SAA are statistically significant)
| LTX AR* | 115.60 ± 38.04 µg/ml |
| LTX CLAD | 109.37 ± 56.04 µg/ml |
| LTX INF* | 127.89 ± 38.88 µg/ml |
| LTX NEG | 76.84 ± 39.06 µg/ml |
| CONTROLS | 43.02 ± 25.18 µg/ml |
Fig. 1.
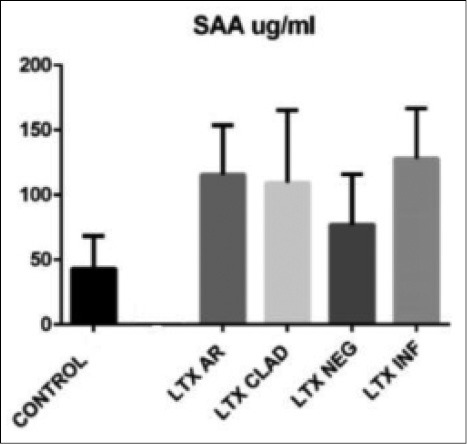
Statistically significant differences found between serum levels of SAA in controls and in LTX patients with acute rejection (LTX AR) (p = 0.01), in patients with acute infection (LTX INF) (p = 0.01). No significant differences were found between the levels of SAA in LTX patients with CLAD and stable transplant patients (NEG) compared to healthy controls
In LTX patients statistically significant correlations were found between SAA levels and the following parameters: C reactive protein (CRP) (r 0.46, p = 0.02); ferritin (r 0.58, p = 0.008); α1-globulins (alpha1%) (r 0.56, p = 0.02); C3 (r 0.56, p = 0.04) and LDL cholesterol (r 0.42, p = 0.06) (Figs. 2 and 3). In AR group, statistically significant correlations were found between SAA serum levels and peripheral CD4+ T-lymphocyte percentages (r 0.9, p = 0.01) and FVC percentages (r -0.94, p = 0.01) (Figs. 4 and 5). Regarding survival analysis, we choose the 75° percentile of SAA levels (8267 ng/ml) as the cut-off value to stratify the population. In our population, patients with SAA concentrations above 75° percentile showed a worse prognosis than those with lower levels, even not significantly (log rank test, p=0.0694; HR 3.68, 95% CI 0.9 to 15.06) (Fig. 6).
Fig. 2.
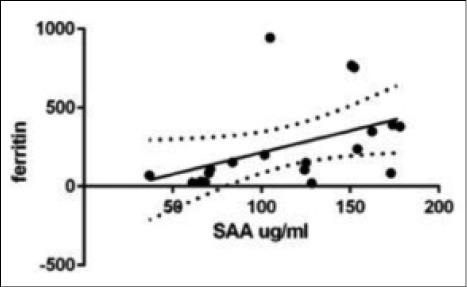
Positive statistical correlation between SAA and Ferritin values in LTX patients (r 0.58, p = 0.008).
Fig. 3.
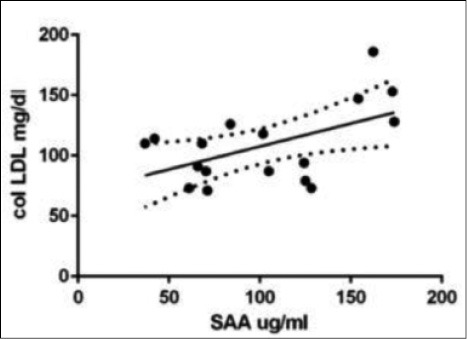
Positive correlation between SAA and LDL cholesterol (with LDL) in LTX patients (r 0.42, p = 0.06).
Fig. 4.
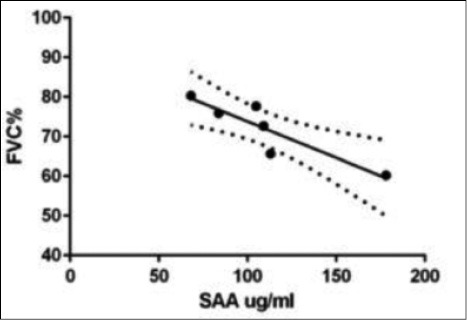
Negative statistical correlation between SAA and FVC (% of predicted) values in LTX patients with acute rejection (r -0.94, p = 0.01).
Fig. 5.
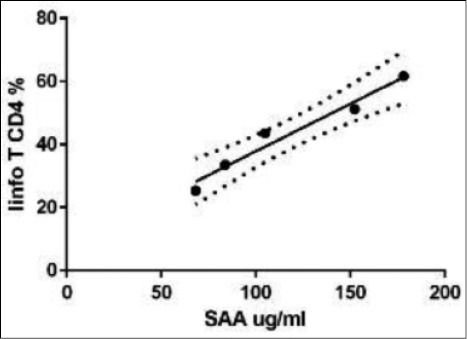
Graphical representation of the statistical correlation between SAA and CD4 + T lymphocytes of peripheral venous blood (lymphoid T CD4%) in lung transplanted patients with acute rejection (r 0.9, p = 0.01).
Fig. 6.
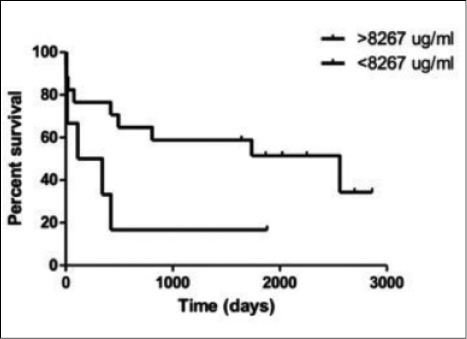
Kaplan-Meier curves for the comparison of survival time in a population of LTX patients, stratified according the 75° percentile of SAA levels.
Discussion
SAA is a pleiotrophic protein involved in the regulation of inflammatory processes as well as in lipid metabolism (21, 22). Its concentrations is increased in serum of patients with chronic inflammatory lung diseases, sarcoidosis for example. Its role as a potential biomarker of different lung diseases has been established, although no data is available on LTX patients (26-28). In our study SAA concentrations were evaluated in LTX to contribute to the definition of novel biomarkers of clinical utility in this field. Serum levels of SAA, as expected, correlated with other acute phase proteins such as CRP, C3, alpha1-globulin and ferritin concentrations, bio-indicators already demonstrated to interfere with inflammatory and infective mechanisms occurring in LTX patients.
Although the small sample size and monocentric design of the study could influence the potential interpretation of the results about the role of SAA in predicting post-transplant complications, our results showed SAA concentrations higher in AR and INF subgroups than in stable LTX patients and controls. This bioindicator was overexpressed in patients with acute complications as a consequence of proinflammatory events, occurring in particular during AR (23). AR patients presented the highest SAA values, that were also correlated with peripheral CD4+ lymphocyte percentages. This data supported the crucial role of CD4+ T cells in AR pathogenesis through a potential SAA signaling that may activate macrophages and overproduction of different acute phase proteins (24). It can be hypothesized that increased SAA levels induce proinflammatory CD4+ cells functions promoting AR. Despite small sample size of our study, outcome analysis was performed and showed a trend between elevated SAA levels and mortality. This data, event if not significant, is interesting and may be driven by the increase of SAA in AR or ongoing infections in LTX, that are both associated with a worse outcome (7). To clarify this point, our data needs to be validated in a larger cohort of patients with a prospective multicenter study focused on a single type of lung transplant complication such as acute rejection.
Moreover, an inverse correlation was observed between SAA levels and FVC percentages in AR patients, suggesting a prognostic role of SAA in acute complications of LTX, rather than a diagnostic biomarker.
In conclusion, SAA resulted an intriguing molecule with a potential prognostic value in patients with AR or infections after lung transplantation.
Abbreviations
- serum amyloid A (SAA)
- lung transplantation (LTX)
- acute rejection (AR)
- acute infection (INF)
- stable patients (NEG)
Biography
L.V. and E.B. conceived of the presented idea, D.B. and A.F. developed the theory, P.C., M.d. and L.B. performed the computations, P.P and L.L discussed the result and contributed to the final manuscript, F.G. and M.M. performed the radiological evaluations and designed the study, D.S. and P.S. planned the study and the analysis, P.R. encouraged the authors contributed to the final version.
References
- 1.Christie JD, Edwards LB, Aurora P, et al. Registry of the international Society for heart and lung tranplantation: twenty-fifth official adult lung and heart-lung transplantation report 2008. J Heart Lung Transplant. 2008;27:957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Egan TM, Murray S, Bustami RT, et al. Development of the new Lung Allocation System in the Unisted States. Am J Trasplant. 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection. J Heart Lung Transplant. 2007:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Yousen SA. Lymphocytic bronchitis/bronchiolitis in lung allograft recipients. Am J Surg Pathol. 1993;17:491–496. doi: 10.1097/00000478-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Boehler A, Estenne M. Post-transplant bronchiolitis obliterans. Eur Respir J. 2003;22:1007–1018. doi: 10.1183/09031936.03.00039103. [DOI] [PubMed] [Google Scholar]
- 6.Glanville AR. Bronchoscopic monitoring after lung transplantation. Semin Respir Crit Care Med. 2010;31:208–221. doi: 10.1055/s-0030-1249117. [DOI] [PubMed] [Google Scholar]
- 7.Avlolnitis VS, Krause A, Luzzi L, et al. Bacterial colonization of the donor lower airways is a predictor of poor outcome in lung transplantation. Eur J Cardiovasc Surg. 2003;24:601–607. doi: 10.1016/s1010-7940(03)00454-8. [DOI] [PubMed] [Google Scholar]
- 8.Orens JB, et al. International guidelines for the selection of lung transplant candidates: 2006 update a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. The Journal of heart and lung transplantation. 2006:744–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Nunley DR, Ohori P, Grgurich WF, et al. Pulmonary aspergillosis in cystic fibrosis lung transplant recipients. Chest. 1998;114:1321–1329. doi: 10.1378/chest.114.5.1321. [DOI] [PubMed] [Google Scholar]
- 10.Bauer Y, White ES, de Bernard S, Cornelisse P, Leconte I, Morganti A, et al. MMP-7 is a predictive biomarker of disease progression in patients with idiopathic pulmonary fibrosis. ERJ Open Res. 2017;3(1) doi: 10.1183/23120541.00074-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan F, Wen Z, Wang R, et al. Identification of the lipid biomarkers from plasma in idiopathic pulmonary fibrosis by Lipidomics. BMC Pulm Med. 2017;17:174. doi: 10.1186/s12890-017-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milara J, Navarro R, Juan G, et al. Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax. 2012;67:147–56. doi: 10.1136/thoraxjnl-2011-200026. [DOI] [PubMed] [Google Scholar]
- 13.Husby G, Natvig JB. A serum component related to non immunoglobulin amyloid protein as a possible precursor of the fibrils. J Clin Inv. 1974;53:1054–61. doi: 10.1172/JCI107642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuroda T, Tanabe N, Hasegawa E, et al. Significant association between renal function and area of amyloid deposition in kidney biopsy specimens in both AA amyloidosis associated with rheumatoid arthritis and AL amyloidosis. Amyloid. 2017;24:123–30. doi: 10.1080/13506129.2017.1338565. [DOI] [PubMed] [Google Scholar]
- 15.Yarur AJ, Quintero MA, Jain A, Czul F, Barkin JS, Abreu MT. Serum Amyloid A as a Surrogate Marker for Mucosal and Histologic Inflammation in Patients with Crohn’s Disease. Inflamm Bowel Dis. 2017;23:158–64. doi: 10.1097/MIB.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 16.Choong CK, Meyers BF, Guthrie TJ, et al. Does the presence of preoperative mild or moderate coronary artery disease affect the outcomes of lung transplantation? Ann Thorac Surg. 2006;82:1038–1042. doi: 10.1016/j.athoracsur.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Vietri L, Bennett D, Cameli P, Bergantini L, et al. Serum amyloid A in patients with idiopathic pulmonary fibrosis. Respiratory Investigation. 2019 Apr 26;S2212-5345(19):30051–6. doi: 10.1016/j.resinv.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Rosas IO, Richards TJ, Konishi K, Kaminski N. MMP1 and MMP7 as Potential Peripheral Blood Biomarkers in Idiopathic Pulmonary Fibrosis. PLoS Med. 2008;5:e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benditt EP, Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci U S A. 1977;74:4025–8. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salazar A, Maña J, Fiol C, et al. Influence of serum amyloid A on the decrease of high density lipoprotein-cholesterol in active sarcoidosis. Atherosclerosis. 2000:497–502. doi: 10.1016/s0021-9150(00)00368-3. [DOI] [PubMed] [Google Scholar]
- 21.Pallotti F, Barassi A, Melzi d’Eril G. La siero amiloide A: biologia ed applicazioni cliniche. Riv Med Lab-JLM. 2003;4(1) [Google Scholar]
- 22.Steinmetz A, Hocke G, Saile R, Puchois P, Fruchart JC. Influence of serum amyloid A on cholesterol esterification in human plasma. Biochim Biophsy Acta. 1989;1006:173–8. doi: 10.1016/0005-2760(89)90192-6. [DOI] [PubMed] [Google Scholar]
- 23.Jensen LE, Whitehead AS. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem J. 1998;334:489–503. doi: 10.1042/bj3340489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Q, Gupta PK, Suzuki H, Wagner SR, Zhang C, Cummings OW. CD4 T cells but not Th17 cells are Required for Mouse Lung Transplant Obliterative Bronchiolitis. Am J Transplant. 2015 Mar 13 doi: 10.1111/ajt.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dew MA, DiMartini AF, Dobbels F, et al. The 2018 ISHLT/APM/AST/ICCAC/STSW recommendations for the psychosocial evaluation of adult cardiothoracic transplant candidates and candidates for long-term mechanical circulatory support. J Heart Lung Transplant. 2018 Jul;37(7):803–823. doi: 10.1016/j.healun.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Sung H-J, Ahn J-M, Yoon Y-H, et al. Identification and validation of SAA as a potential lung cancer biomarker and its involvement in metastatic pathogenesis of lung cancer. J. Proteome Res. 2011;10:1383–1395. doi: 10.1021/pr101154j. [DOI] [PubMed] [Google Scholar]
- 27.Chen ES, Song Z, Willett MH, et al. Serum amyloid A regulates granulomatous inflammation in sarcoidosis through Toll-like receptor-2. Am. J. Respir. Crit. Care Med. 2010;181:360–373. doi: 10.1164/rccm.200905-0696OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bozinovski S, Hutchinson A, Thompson M, et al. Serum amyloid a is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008;177:269–278. doi: 10.1164/rccm.200705-678OC. [DOI] [PubMed] [Google Scholar]


