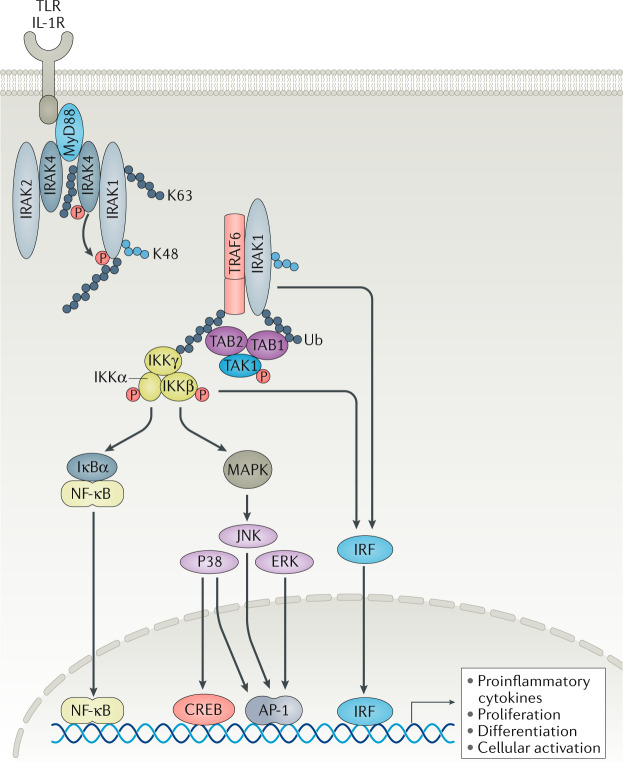
Fig. 3. IRAK4 is the upstream kinase that transduces TLRs and IL-1R signals.
IL-1 receptor (IL-1R)-associated kinase 1 (IRAK1) and IRAK4 function is regulated by protein–protein interactions and by post-translational modifications that may be uniquely regulated in different cell types to fine-tune an immune response. IL-1R or Toll-like receptor (TLR; except for TLR3) engagement causes the recruitment of adaptor protein myeloid differentiation primary response 88 (MyD88) to the intracellular Toll/IL-1R receptor (TIR) domains to initiate the Myddosome assembly. MyD88 recruits IRAK4 via death domain interactions. IRAK4 is activated via autophosphorylation and is also K63-ubiquitylated (grey circles). Phosphorylated IRAK4 can activate IRAK1, which facilitates IRAK1–tumour necrosis factor receptor-associated factor 6 (TRAF6) complex formation. K48 ubiquitylation (blue circles) of IRAK1 is required for the activation of TGFβ-activated kinase 1-binding protein 1 (TAK1), and its binding to TAB1 and TAB2 to drive the formation of the inhibitor of NF-κB (IKK) complex and subsequent NF-κB inhibitor-α (IκBα) activation, which then leads to NF-κB, mitogen-activated protein kinase (MAPK) and interferon-regulatory factor (IRF) activation to induce the transcription of pro-inflammatory cytokines and cellular processes, such as proliferation and activation61,62. The mechanism of IRF activation by MyD88 is less understood. Downstream of IRAK4 activation, TAK1 and IKKβ complex can mediate IRF5 phosphorylation, nuclear translocation and transcription in monocytes74, but IRAK1 can also directly bind and phosphorylate IRF7 in plasmacytoid dendritic cells263. The mechanism of IRAK4 kinase activity-independent action is less understood but may involve K63-ubiquitylated signalling hubs and other novel molecular scaffolds92. AP-1, activator protein 1; CREB, cAMP response element-binding protein; P, phosphorus; TAB, TGFβ-activated kinase 1 binding protein; Ub, ubiquitin.