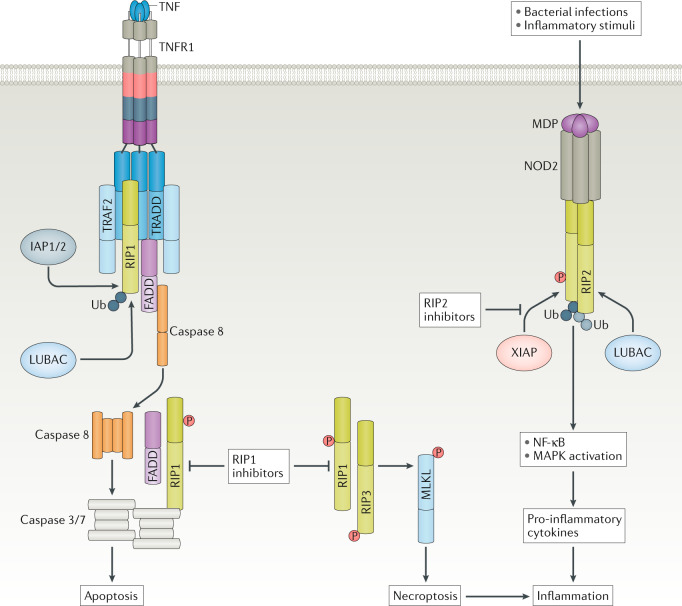
Fig. 4. RIP kinases regulate cell death and inflammatory pathways.
Tumour necrosis factor (TNF) signalling can lead to receptor-interacting protein 1 (RIP1)-dependent apoptosis (mediated by caspase 8) or necroptosis (mediated by RIP3 and mixed-lineage kinase domain-like protein (MLKL)) to cause tissue damage and an inflammatory milieu. RIP1 inhibition can block RIP1-mediated apoptosis and necroptosis, and reduce inflammation by inhibiting inflammatory cell death. The kinase domain of RIP2 allows the binding of E3 ligase X-linked inhibitor of apoptosis protein (XIAP) and subsequent RIP2 ubiquitylation, which is a critical mediator of nucleotide-binding oligomerization domain-containing protein 2 (NOD2) inflammatory signalling. Consequently, RIP2 kinase inhibitors prevent XIAP binding and RIP2 ubiquitylation to inhibit NOD2 pathway-activated NF-κB and mitogen-activated protein kinase (MAPK) signalling, and consequent production and release of pro-inflammatory cytokines, thus blocking inflammation. FADD, FAS-associated death domain; IAP1/2, inhibitor of apoptosis 1 and 2; LUBAC, linear ubiquitin chain assembly complex; MDP, muramyl dipeptide; P, phosphorus; TNFR1, TNF receptor 1; TRADD, TNFR-associated death domain; TRAF2, TNFR-associated factor 2; Ub, ubiquitin.