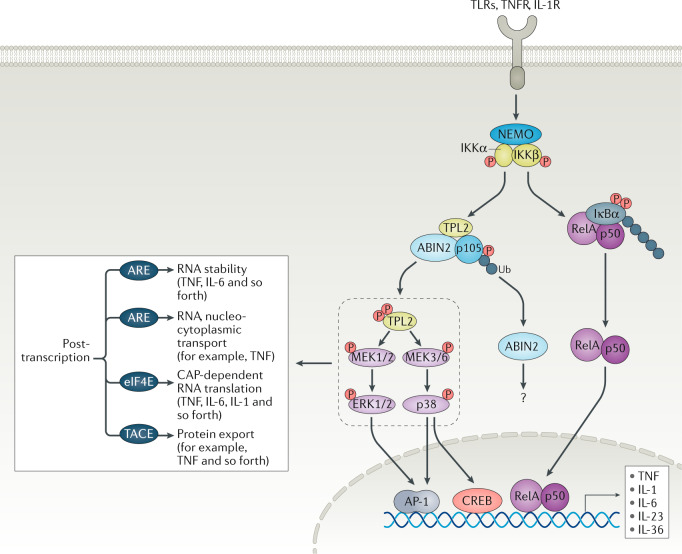
Fig. 6. TPL2 regulatory inflammatory response downstream of TLRs, TNFR and IL-1R.
The action of both mitogen-activated protein kinase (MAPK) and NF-κB orchestrates the transcription of target genes. In the resting state, p105 prevents and masks tumour progression locus 2 (TPL2) kinase effector function. Agonist stimulation activates the inhibitor of NF-κB (IKK)/NF-κB essential modulator (NEMO) complex264. Subsequently, IKKβ phosphorylates IKKα, targeting IKKα for proteasomal degradation. Then, released RelA/p50 dimers are translocated to the nucleus and modulate target gene expression. IKKβ also phosphorylates the target residues S927 and S932 in p105, leading to its proteasomal degradation that results in TPL2 liberation. IKKβ phosphorylates TPL2 at residue S400 to enhance its kinase activity. Free TPL2 then activates MEK1 and MEK2 as well as MEK3 and MEK6 to positively regulate ERK1/2 or p38α/δ to regulate gene transcription via cAMP response element-binding protein (CREB)/activator protein 1 (AP-1) as well as mRNA stability and protein production. The function of A20-binding inhibitor of NF-κB (ABIN2) is not completely understood and involves regulation of protaglandin E2 (PGE2) and cyclooxygenase 2 (COX2) in fibroblasts. TPL2 binds to a small fraction of p105 but the p50 domain of processed p105 can directly impact gene transcription16. Post-transcriptional regulation of cytokines and chemokines by MAPKs involves AU-rich elements (AREs) on messenger RNAs to dictate their stability (in the case of tumour necrosis factor (TNF) or IL-6, for instance) or cellular localization (TNF). In addition, other regulatory processes such as CAP-dependent RNA translation and protein export via TNFα-converting enzyme (TACE) (in the case of TNF) are regulated by the TPL2/MAPK axis. Therefore, the net effect of TPL2 inhibition has a profound effect on inflammatory outputs without compromising the NF-κB pathway. IL-1R, IL-1 receptor; P, phosphorus; TLR, Toll-like receptor; TNFR, TNF receptor; Ub, ubiquitin.