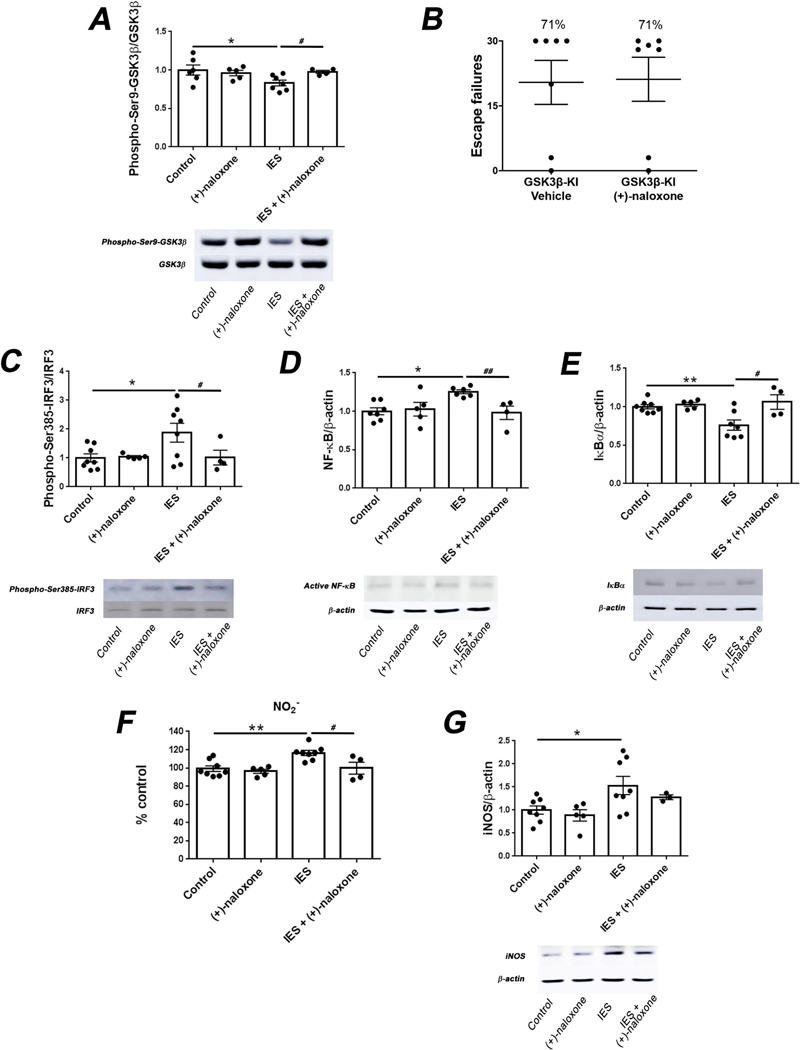
Fig. 2.
Administration of (+)-naloxone inhibits the TLR4 signaling pathway induced by stress. (A) Wild-type non-shocked (Control) mice or mice subjected to inescapable foot shocks (IES) were administered the TLR4 antagonist (+)-naloxone (5 mg/kg; i.v.) or vehicle 1 h prior to IES and sacrificed 12 h after IES. Hippocampal levels of phospho-Ser9-GSK3β and total GSK3β were measured. Each point represents an individual mouse. Bars represent means ± SEM. One-way ANOVA, F(3,18) = 3.004, Dunnett post-hoc test, *p < 0.05, compared to control mice, Student’s t-test, t = 2.728, #p < 0.05, IES vs. IES+ (+)-naloxone, n = 4–7 mice/group. (B) GSK3β knockin (GSK3β-KI) mice were treated with (+)-naloxone (5 mg/kg; i.v.) or vehicle and subjected to the learned helplessness paradigm 1 h after treatment. The number of failures to escape was recorded. Mice were considered learned helplessness if they failed to escape more than 15 out of 30 trials of escapable foot shocks. Each point represents the number of failures to escape for an individual mouse. Bars represent means ± SEM. n = 7 mice/group. (C-G) Wild-type non-shocked (Control) mice or mice subjected to inescapable foot shocks (IES) were administered (+)-naloxone (5 mg/kg; i.v.) or vehicle 1 h prior to IES and sacrificed 12 h after IES. Hippocampal levels of (C) phospho-Ser385-IRF3 and total IRF3, (D) active NF-κB and β-actin, (E) IκBα and β-actin, (F) NO2−, a stable breakdown product of NO, and (G) iNOS and β-actin were quantified. Each point represents an individual mouse. Bars represent means ± SEM. One-way ANOVA, (C) F(3,21) = 3.507, (D) F(3,18) = 4.620, (E) F(3,20) = 6.776, (F) F(3,21) = 7.994, (G) F(3,20) = 3.904, Dunnett post-hoc test, *p < 0.05, **p < 0.01, compared to control mice, Student’s t-test, (C) t = 4.917, (D) t = 3.578, (E) t = 2.673, (F) t = 2.817, #p < 0.05, ##p < 0.01, IES vs. IES + (+)-naloxone, n = 3–8 mice/group.