Table 1.
Synthesis and inhibitory activity of 2-(isopropylamino)thiazol-4(5H)-one derivatives.
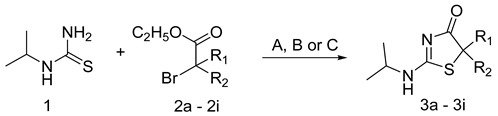
| No | R1 | R2 | Procedure Time (h) | Isolated Yield (%) | M.p. (°C) | % of 11β-HSD1 Inhibition 10 μM/IC50 [µM] a | % of 11β-HSD2 Inhibition 10 μM/IC50 [µM] a |
|---|---|---|---|---|---|---|---|
| 3a | H | CH3 | A/7 | 34 | 115–117 | 0 | 27.61 ± 2.11/nd |
| 3b | H | C2H5 | A/8 | 28 | 130–131 | 0 | 47.08 ± 2.51/nd |
| 3c | H | n-C3H7 | A/10 | 47 | 110–112 | 0 | 54.59 ± 4.21/9.12 ± 0.76 |
| 3d | H | CH(CH3)2 | A/15 | 25 | 153–155 | 18.86 ± 3.47/nd | 14.29 ± 1.21/nd |
| 3e | CH3 | CH3 | A/11 | 10 | 193–194 | 18.06 ± 0.97/nd | 21.43 ± 2.11/nd |
| 3f | H | C6H5 | B/240 | 25 | 227 (dec.) | 0 | 10.48 ± 2.13/nd |
| 3g | H | pBr-C6H4 | B/168 | 85 | 236–237 | 27.58 ± 2.53/nd | 27.61 ± 2.11/nd |
| 3h | C5H10cycl | C/168 | 8 | 140 (dec.) | 54.53 ± 3.03/9.35 ± 0.67 | 17.35 ± 1.25/nd | |
| 3i | C3H6cycl | C/240 | 15 | 151–152 | 20.94 ± 2.22/nd | 36.73 ± 2.43/nd | |
| control | 84.54 ± 5.47 b/<0.625 | 47.43 ± 1.11 c/nd | |||||
Procedure A: MeOH, MeONa; reflux, Procedure B: CHCl3, RT; Procedure C: EtOH, DIPEA, reflux, nd —Not determined due to too low % enzyme inhibition value (the cutoff value for this assay was 50% inhibition at the inhibitor concentration of 10 µM), a values were obtained from three independent determinations, IC50 were determined with 5 inhibitor concentrations, b for carbenoxolone, c for 18β-glycyrrhetinic acid.
