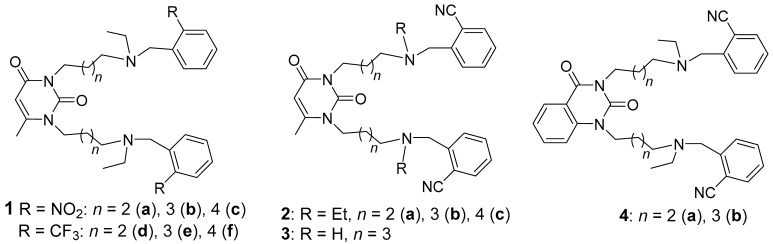
Table 1.
In vitro inhibition of AChE from human erythrocytes and serum hBChE by compounds with 6-methyluracil, quinazoline-2,4-dione moieties, varied polymethylene chains, amino groups, and substituents at benzene rings compared to donepezil hydrochloride a.
| Compound | IC50 [nM] | AChE Selectivity b | LD50, mg/kg c | |
|---|---|---|---|---|
| AChE | BChE | |||
| 1a d | 47 ± 3 | 50,000 ± 3000 | 1064 | 281 |
| 1b d | 3.5 ± 0.3 | 35,000 ± 500 | 10,000 | 51 |
| 1c d | 83 ± 10 | 90,000 ± 3000 | 1084 | 93 |
| 1d d | 67 ± 8 | 20,000 ± 150 | 298 | 59 |
| 1e d | 5.6 ± 0.7 | 20,000 ± 2000 | 35,714 | 49 |
| 1f d | 1400 ± 90 | 200,000 ± 2000 | 143 | 58 |
| 2a | 7 ± 0.5 | 65,000 ± 500 | 9285 | 284 |
| 2b | 7.3 ± 0.6 | 100,000 ± 12,000 | 14,286 | 138 |
| 2c | 29 ± 0.3 | 10,000 ± 150 | 345 | 282 |
| 3 | 480 ± 30 | 1800 ± 170 | 3.8 | 146 |
| 13 | 2320 ± 170 | 2160 ± 150 | 0.9 | 10 |
| 4a | 5520 ± 400 | 2620 ± 200 | 0.5 | 107 |
| 4b | 38 ± 3 | 30,000 ± 500 | 789 | 1.1 |
| 2b·2HBr | 1.7 ± 2 | 942 ± 12 | 5541 | 13.1 |
| 2c·2HBr | 24 ± 2 | 10,000 ± 700 | 417 | 11.3 |
| 4b·2HBr | 46 ± 5 | 3920 ± 200 | 85 | 265 |
| Donepezil | 48 ± 3 | 7900 ± 600 | 165 | 5 |
IC50 values are expressed as mean of three independent measurements, each performed in duplicate; a the acetylthiocholine and butyrylthiocholine concentration was 1 millimole/L (mM); b (IC50hBChE)/(IC50hAChE); c mice, intraperitoneal injection; d data for compounds 1a–f were taken from reference [25].