Fig. 1.
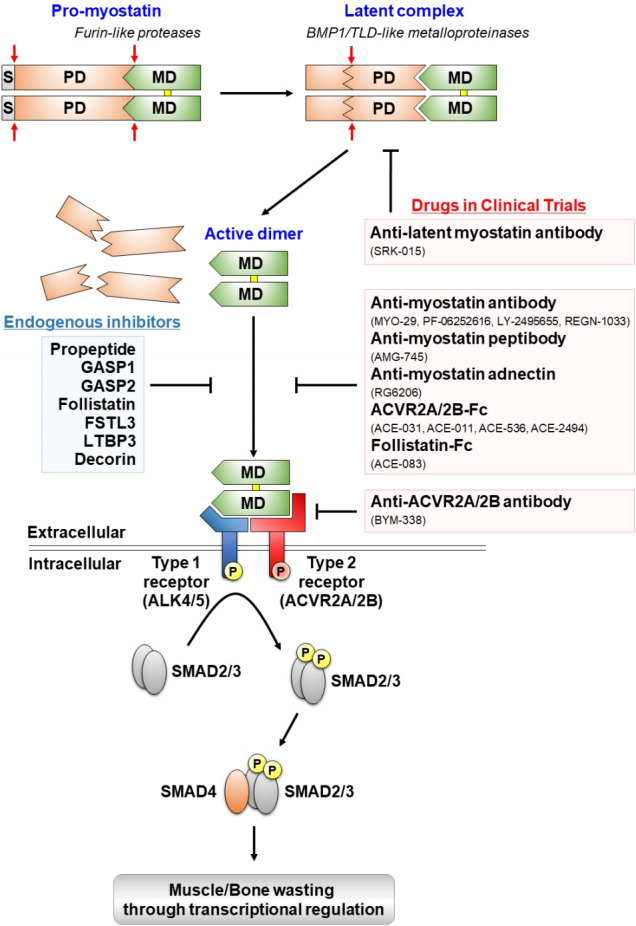
Proteolytic processing, extracellular regulation, and signaling mechanisms of myostatin and myostatin-targeting drugs in clinical trials. Myostatin is first synthesized as a precursor molecule (pro-myostatin) that undergoes proteolytic processing to produce the biologically active molecule. After removal of the signal peptide (S), pro-myostatin is cleaved by a furin-like protease to form a latent complex, leaving the mature domain (MD) non-covalently attached to the prodomain (PD). The latent complex is further cleaved by a bone morphogenetic protein 1 (BMP1)/ tolloid (TLD)-like metalloproteinase to generate the mature, disulfide-linked (marked in yellow), dimer that initiates signal transduction. The mature myostatin ligand binds to constitutively phosphorylated activin type 2A and 2B receptors (ACVR2A/2B) that subsequently recruit and phosphorylate (P) activin receptor-like kinase 4 and 5 (ALK4/5), leading to phosphorylation and activation of Smad2/3. Finally, activated Smad2/3 assembles with Smad4 and translocates to the nucleus to regulate expressions of target genes associated with muscle and bone homeostasis. Endogenous inhibitors of myostatin and myostatin-targeting drugs in clinical trials are indicated by blue and red boxes, respectively.
