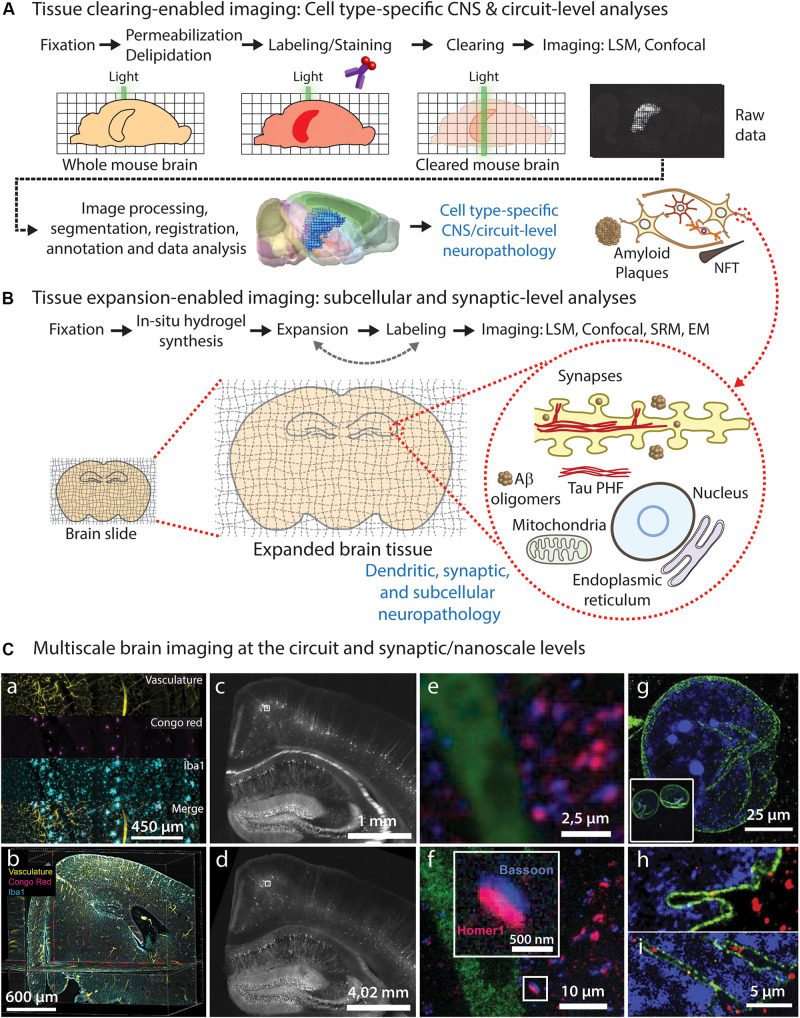
FIGURE 1.
Brain tissue clearing and expansion approaches for neuropathological imaging. (A) Common steps required for whole-brain tissue clearing, imaging, and analysis of neuropathological features, allowing CNS-wide identification of vulnerable circuits and cell types. (B) Tissue expansion approaches can be used to study early pathological alterations caused by abnormal protein conformation, aggregation, and subsequent ultrastructural changes in specific subcellular compartments and organelles within affected cell types and circuits identified using tissue clearing. (C) Diffraction-limited light microscopy of cleared brain tissue allows imaging of large brain circuits at cellular resolution (a–c), including analysis of neuropathological markers (a,b), but it does not reach synaptic nanoscale resolution in non-expanded tissues (e). On the contrary, expansion microscopy of tissues and cells (d,f-i) allows nanoscale imaging using diffraction-limited microscopes, enabling detailed visualization of synapses (e) and other subcellular structures such as nuclear envelope invaginations (g–i) in healthy (h) and ALS pathological conditions (i). (a,b) LSFM images showing individual channels (a) and orthogonal optical planes (b) of a cleared transgenic AD mouse brain after staining of vasculature (yellow), Aβ plaques (Congo red; magenta) and microglia (Iba1; cyan) using the iDISCO protocol; adapted from Liebmann et al. (2016), with permission from Elsevier. (c-f) Epifluorescence images of a Thy1-YFP mouse brain stained with presynaptic (Bassoon, blue) and postsynaptic (Homer1, red) markers, before (c,e) and after (d-f) expansion microscopy; adapted from Chen et al. (2015), with permission from AAAS. (g-i) Confocal microscopy images of expanded human iPSC-derived motor neuron nuclei from healthy control (h) and ALS patient (i) [(g) inset: nuclear size before expansion], immunolabeled for LMNB1 (green) and DNA (blue); adapted from Ortega et al. (2020), with permission from Elsevier.