Abstract
Background
The outbreak of the COVID-19 pandemic facilitated a rapid transition to non–face-to-face models of care across the allergy services.
Objective
To describe the outcomes of the use of synchronous telemedicine for outpatient consultations in a tertiary adult allergy center.
Methods
We retrospectively reviewed all non–face-to-face appointments during the second month of the pandemic in the United Kingdom.
Results
A total of 637 non–face-to-face appointments for unique patients were booked between April 1 and 30, 2020; 91% were new consultations. Most referrals (81.5%) were related to nondrug reactions. The overall “Did Not Attend” rate was 15.7%. A total of 439 patients were assessed for nondrug reactions; 87% were new appointments. Food-related reactions (50.4%), urticaria/angioedema (23.2%), and rhinitis (18.1%) were the most common reasons for new referrals. Two hundred twenty-one (57.7%) of these patients required further allergy testing, primarily for suspected food allergy. More than 42% of the new patients, mainly referred for urticaria/angioedema, were discharged after their remote assessment. Less than 10% of the follow-up patients required additional testing. Ninety-seven new patients were assessed for a suspected drug reaction, predominantly to beta-lactam antibiotics (57.7%). Sixty-nine patients (71%) required further investigations, but a notable 29% did not require further allergy input. The overall experience was very good/good for most patients (85%).
Conclusion
Telemedicine can transform the current models of allergy care. Screening criteria for selecting suitable new patients are required. A telemedicine-based drug allergy service model can be more time- and cost-effective, and improve patient access to specialist care.
Key words: Telemedicine, Allergy, Synchronous, Service model, COVID-19
Abbreviations used: ADR, Adverse drug reaction; DNA, Did Not Attend; f2f, Face-to-face; Nf2f, Non–face-to-face; NHS, National Health Service; non-ADR, Non–adverse drug reaction; TM, Telemedicine
What is already known about this topic? Telemedicine is an innovative tool that can transform the current models of allergy care. The COVID-19 pandemic introduced the potential of synchronous telemedicine across the allergy services.
What does this article add to our knowledge? In an unselected patient cohort, a significant number of new allergy consultations (42% non–drug-related; 29% drug-related) were completed without an in-person visit or allergy testing. Less than 10% of the follow-up patients required additional testing.
How does this study impact current management guidelines? Screening criteria for selecting suitable new patients for non–face-to-face appointments are required. A telemedicine-based drug allergy service model can result in time and cost savings, while improving patient access to specialist care.
Telemedicine (TM) is defined as the use of electronic communications and information technologies to provide remote clinical services to patients without an in-person visit.1 TM has become increasingly popular over the last few years due to its immense potential to positively impact a wide range of populations and address major barriers in access to care.2, 3, 4 The non–face-to-face (Nf2f) models can be categorized as either synchronous or asynchronous. Synchronous interactions occur in “real time” between the patient and the health care provider, and consist of a prebooked audio-only (telephone) or 2-way video session. In the asynchronous Nf2f care model, the patient and the provider are not online at the same time. The 2 types of asynchronous care include “store & forward” applications and remote patient monitoring.2
Although the use of TM in allergy is not new,5, 6, 7 it was only after the outbreak of the COVID-19 pandemic and following the advice from Public Health England and the UK government to “stay at home” and avoid nonessential travel that the adult allergy services across the United Kingdom transitioned to an Nf2f model almost overnight to reduce or replace face-to-face (f2f) visits.
We report on the outcomes of the use of synchronous TM for outpatient allergy consultations in a tertiary adult allergy center. This is the first study with a completely unselected patient cohort. Because of the pandemic and the unexpected switch from in-person visits to Nf2f clinics, the patients had not been offered the choice to have an f2f or an Nf2f appointment. The referral letters had not been previously reviewed to decide if a remote consultation was appropriate. Moreover, on the basis of our experience, we propose a new model of care that integrates TM into a drug allergy service.
Methods
We retrospectively reviewed all Nf2f clinic appointments in the Department of Adult Allergy, Guy's and St Thomas' National Health Service (NHS) Foundation Trust, London, United Kingdom, which took place between April 1 and 30, 2020 (second month of the pandemic in the United Kingdom). All Nf2f appointments were synchronous and conducted by telephone. The clinicians had full access to the Electronic Patient Records (iSOFT Group Plc, Aldershot, Hampshire, UK) and an electronic medical notes system to document the consultation. The allocated duration of an Nf2f appointment was 20 minutes. The clinical team included eight allergy consultants (specialists) and 2 specialty registrars (residents in training). The referral letters had been previously vetted, and the patients were deemed appropriate for an f2f allergy review.
We determined the number of new and follow-up appointments and the overall Did Not Attend (DNA) rate. The patients who did not have a consultation were not included in the analysis. We reviewed the reasons for the referral based on the information provided by the referring clinician (all new appointments) and the working diagnosis from the previous allergy review (follow-up appointments). On the basis of the reason for the referral, we divided the Nf2f appointments into 2 groups: related to adverse drug reactions (ADR) and non–adverse drug reactions (non-ADR). We then divided each into new and follow-up appointments.
We identified the Nf2f clinic outcomes and the number of patients who required further f2f allergy testing after their remote assessment. We identified how many needed to return to our department for testing and how many were advised to have the diagnostic tests organized by the referring clinician. Finally, we determined how many follow-up appointments were requested, and if a referral to a different specialty was required.
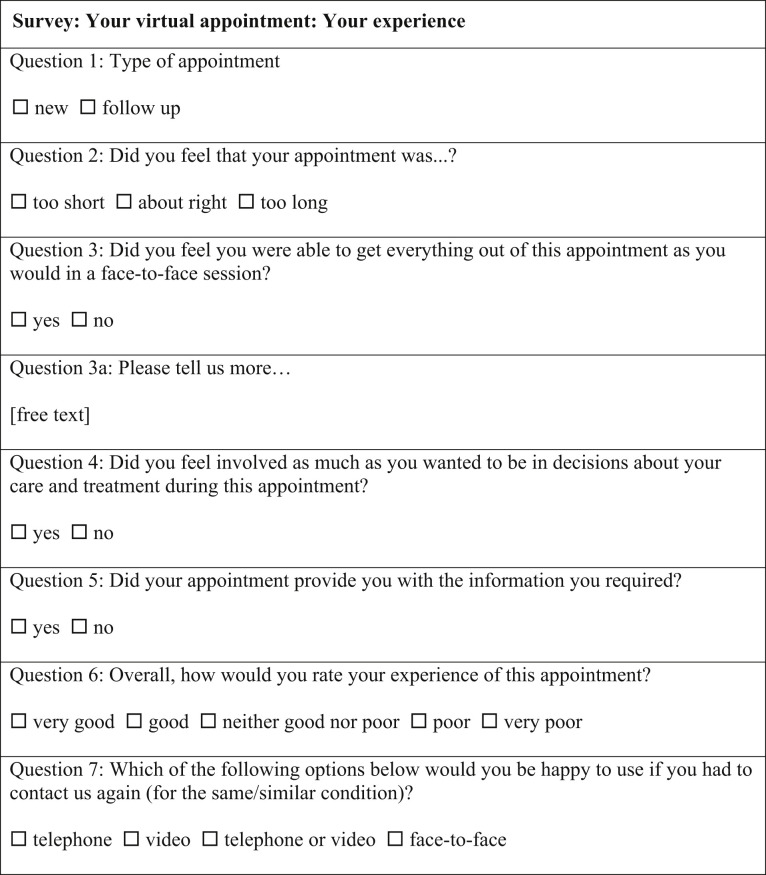
Patients' feedback was collected after their appointment by the Patient Engagement team using an anonymous electronic survey (see Figure E1 in this article's Online Repository at www.jaci-inpractice.org). Descriptive statistics were used to summarize the results. This study was conducted as part of an approved service evaluation within the department.
Figure E1.
Survey: your virtual appointment: your experience.
Results
A total of 637 Nf2f appointments were booked for unique patients in April 2020. The majority (91%) were new consultations. The mean age was 38.1 ± 16 years, with age range from 16 to 89 years (Table I ). Two-thirds of patients were female. The majority of referrals (81.5%) were not related to adverse drug reactions (non-ADR); 18.5% were for a suspected ADR.
Table I.
Patients characteristics, type of Nf2f clinic appointments and indications for allergy referrals
| All (n = 637) | DNA (n = 100) | Indications for referral | Non-ADR (n = 439) | Indications for referral | ADR (n = 98) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sex (male:female) | 206:431 | 35:65 | 138:301 | 33:65 | |||||
| Age (y), mean ± SD | 38.1 ± 16 | 38.8 ± 16.6 | 36.3 ± 15.1 | 45.6 ± 17.4 | |||||
| Age range (y) | 16-89 | 17-86 | 16-89 | 17-81 | |||||
| Type of clinic appointment | New (n = 98) | Follow-up (n = 2) | New (n = 383) | Follow-up (n = 56) | New (n = 97) | Follow-up (n = 1) | |||
| Reason for referral, n (%): | Food-related reactions | 193 (50.4) | 21 (37.5) | BL antibiotics | 56 (57.7) | 0 (0) | |||
| Urticaria/angioedema | 89 (23.2) | 26 (46.4) | Non-BL antibiotics | 8 (8.25) | 0 (0) | ||||
| Rhinitis | 69 (18.1) | 5 (8.9) | Local anesthetics | 8 (8.25) | 0 (0) | ||||
| Atopic dermatitis | 10 (2.6) | 1 (1.8) | NSAID | 7 (7.2) | 0 (0) | ||||
| Other | 22 (5.7) | 3 (5.4) | Radiocontrast media | 3 (3.1) | 0 (0) | ||||
| Perioperative reactions | 2 (2) | 0 (0) | |||||||
| Other | 13 (13.5) | 1 (100) | |||||||
ADR, Adverse drug reaction; BL, beta-lactam; DNA, did not attend; non-ADR, non–adverse drug reaction; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation.
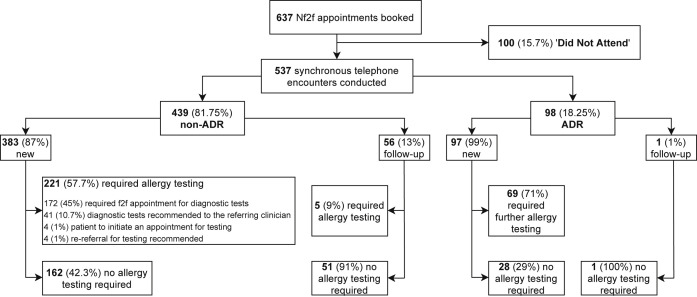
A total of 100 patients “did not attend” their Nf2f appointment (overall DNA rate 15.7%) and were excluded from further analysis (Figure 1 , Table I). The DNA rate was similar across the non-ADR and ADR groups. In the non-ADR group, 97.5% of the patients who ‘did not attend’ were newly referred. Food-related reactions (53.75%), urticaria/angioedema (27.5%), and rhinitis (11.25%) were the most common reasons for referral. All the patients who referred for a suspected ADR and “did not attend” their appointments were new patients. Adverse reactions to antibiotics (45%), mainly beta-lactams, and local anesthetics (22%) were the most common indications in this group (data not shown).
Figure 1.
Non–face-to-face (Nf2f) appointments flowchart. This flowchart illustrates all the Nf2f clinic appointments booked in April 2020, and their outcomes. ADR, Adverse drug reaction.
A total of 537 synchronous telephone encounters were conducted; 439 (81.75%) related to non-ADR and 98 (18.25%) to a suspected ADR (Figure 1). In the non-ADR group, there were 87% new and 13% follow-up appointments. The mean age was 36.3 ± 15.1 years (range, 16-89 years); 68.5% of the patients were female.
The most common non-ADR indication for a new patient to be referred to our service was IgE and/or non–IgE-mediated adverse food reactions (50.4%). Nearly a quarter of the new referrals were related to urticaria/angioedema (23.2%), and 18.1% of the patients were referred for rhinitis assessment. A small number of referrals were related to atopic dermatitis (2.6%) or other indications (5.7%), such as insect venom allergy, idiopathic anaphylaxis, or histamine intolerance (Table I). Of note, the patients with asthma are managed by the respiratory service in our Trust, hence not represented in our cohort.
From the 383 new patients with non-ADR, 221 (57.7%) required further allergy testing (skin and/or blood tests and/or challenge) (Figure 1). For most of the patients (45%), an in-person visit was going to be arranged in our department after the easing of the pandemic-related restrictions. In 10.7% of patients, the referring clinicians were advised to perform the relevant diagnostic tests. A small number of patients (1%) were advised to contact our department to arrange further testing in a few months. A recommendation to re-refer to the department for an f2f assessment and testing after the pandemic was made to the referring clinician in 1% of the patients. The patients requiring allergy testing were predominantly under investigation for suspected food allergy (72%) and rhinitis (19%).
After an Nf2f consultation, more than 42% of the new patients were discharged without requiring further allergy investigations or input. Therefore, no further f2f visit was arranged. These patients had been mainly referred for urticaria/angioedema (49.4%), food-related reactions (21%), and rhinitis (16.6%). Routine laboratory testing (eg, full blood count, thyroid function, etc.) was recommended to the referring physician in 15% of the discharged patients, most of them with urticaria/angioedema (88%). Five of the discharged patients (1.3%) were referred to dermatology, mainly for suspected allergic contact dermatitis, and 2 patients (0.5%) to ear, nose, and throat for further assessment of their rhinitis. Twenty of the newly referred patients (5.2%) would require a follow-up appointment in our department; nearly half of them had been referred for urticaria/angioedema.
A total of 56 patients had a telephone follow-up consultation. Urticaria/angioedema appears to be the most common diagnosis for follow-up appointments (46.4%), followed by food-related reactions (37.5%). Most of the follow-up patients (91%) did not require further allergy investigations, and the majority of them (80%) were fully discharged from the service. Two of these patients (4.5%) were referred to dermatology for further review, 1 for difficult urticaria and 1 for severe atopic dermatitis. Eleven patients (20%) would require a further appointment in our department; 4 of these patients had a diagnosis of complex food allergy, 3 patients were suffering from refractory chronic urticaria, 2 patients were teenagers/young adults initially referred from the transition clinic (a joint pediatric/adult allergy clinic for smooth transition to adult services), and 2 patients would require a challenge in the Day Unit. Routine laboratory testing was recommended to the referring physician after the Nf2f appointment for 1 patient.
Ninety-eight consultations were conducted for suspected ADR; 97 were new patients. The mean age was 45.6 ± 17.4 years (range, 17-81 years); two-thirds of the patients were female. Suspected allergy to beta-lactam antibiotics was the most common reason for referral (57.7%), followed by non–beta-lactam antibiotics (8.25%), local anesthetics (8.25%), nonsteroidal anti-inflammatory drugs (7.2%), radiocontrast media (3.1%), and perioperative reactions (2%) (Table I). The rest of the suspected ADR referrals (13.5%) were related to a heterogeneous group of medications. Sixty-nine patients (71%) required further f2f investigations (eg, skin prick testing, intradermal testing, in vitro testing, drug provocation test, etc.). However, 29% of the patients referred with a suspected ADR did not require further allergy input after their Nf2f review and were discharged (Figure 1). The 1 follow-up patient did not require further investigations either.
One hundred twenty-two of 537 patients (22.7%) submitted anonymous patient satisfaction surveys; two-thirds of the responses were from new appointments. The majority of the patients (91%) felt that the duration of the Nf2f consultation was “about right.” A total of 75% of the patients felt that they were able to get everything out of their Nf2f appointment as they would in an f2f session. Sixty-six (54%) patients provided more information in the free text comment box; the majority of these comments were positive (78%). The most common themes appearing in the negative comments include the need for a future f2f visit to undergo allergy testing, the low audio quality, and the fact that the telephone consultation felt more impersonal than an f2f visit. Most patients (90%) felt involved to the extent they wanted in the decisions about their care and treatment, and adequate information about their diagnosis was provided (87%). The overall experience was very good/good for most patients (85%). However, 32% of the patients would have chosen an f2f appointment if they could for the same or similar condition in the future.
Discussion
The UK NHS has been undergoing a digital transformation, and the existing model of care is expected to look markedly different in 10 years' time. According to the NHS long-term plan,8 one of the major changes to the current NHS service model is the transition to a restructured, digitally enabled outpatient care model. The traditional model of outpatient care appears unsustainable, and the plan is to be redesigned with the goal to reduce the f2f visits up to a third over the next 5 years. The COVID-19 pandemic facilitated a sudden change in the model of care, from the standard f2f visit to widespread adoption of TM across the allergy services.9, 10, 11, 12 Changes that would typically require years of forward planning occurred in no time, and the allergy community was introduced to the transformational potential of TM.
It is important to highlight that all the patients in our study would have been booked for an f2f appointment if it had not been for the pandemic. As mentioned earlier, the patients were unselected and none of the new or follow-up appointments had undergone a suitability screen for an Nf2f visit. Also, unlike previous studies6 , 7 that included pediatric patients as well, all patients were ≥16 years old, which is the cutoff age for adult allergy service in the United Kingdom.
Food-related reactions (50.4%), urticaria/angioedema (23.2%), and rhinitis (18.1%) represented the most common non-ADR reasons for review. More than 42% of the new patients were discharged from the Allergy Service after a single Nf2f consultation. This highlights the fact that a significant number of allergy consultations can be completed without an in-person visit or allergy testing. During an Nf2f consultation, the clinicians are able to engage in a conversation similar to an f2f visit and obtain a comprehensive allergy history. If required, patients can provide more information after the Nf2f appointment: images of the rash, previous laboratory results or discharge summaries from emergency department visits, using a secure email address provided by the clinician. Patient information leaflets and other educational materials could be easily provided via a validated web link in the clinic letter that follows the Nf2f appointment; alternatively, these links could be available on the department's website. Nearly half of the discharged patients had been referred for what proved to be spontaneous urticaria/angioedema, where the diagnosis is based primarily on the clinical presentation, and allergy testing is not usually indicated, as per the current guidelines from the European Academy of Allergy and Clinical Immunology13 and the American Academy of Allergy, Asthma and Immunology.14 Other scenarios where allergy testing might not be necessary (38% of our cohort) included pollen food syndrome in patients with confirmed seasonal allergic rhinitis, IgE-mediated food allergy with the relevant in vitro tests already performed in the community, non–IgE-mediated food-related reactions (eg, food intolerances), and allergic rhinitis recently investigated in primary care.
A total of 10.7% of the new non-ADR patients, referred mainly for suspected food allergy and seen during the pandemic, were discharged back to primary care with a recommendation for diagnostic in vitro allergy tests. In normal (nonpandemic) times, some primary care physicians might be reluctant to perform allergy tests (specific IgE) as they are not trained to interpret them. Moreover, some UK NHS primary care authorities do not allow them in primary care due to cost.
TM definitely has a role to play in the management of new referrals for non-ADR. Relevant models of care in allergy have been previously suggested.15 However, more than half of these patients in our cohort required a further f2f visit for allergy testing, mostly for suspected food allergy and rhinitis. Therefore, detailed screening criteria should be in place to identify patients suitable for a remote assessment (Table II ). These criteria could be applied during the referral vetting process for Nf2f patient selection. The UK General Medical Council have also produced generic criteria to help determine whether a remote consultation is appropriate.16
Table II.
Allergy specific vetting criteria for identifying suitable new referrals for remote consultation
| Non–adverse drug reactions | Adverse drug reactions |
|---|---|
| 1. Urticaria/angioedema | 1. Suspected drug-induced∗ HR (immediate or delayed) |
| 2. Presumed allergic reaction with unclear history | 2. Suspected ACEi-induced angioedema |
| 3. Suspected idiopathic anaphylaxis | 3. Suspected NSAID hypersensitivity |
| 4. Suspected pollen food syndrome in patients with confirmed seasonal allergic rhinitis | 4. CRSwNP, referred for consideration of aspirin desensitization |
| 5. Suspected IgE-mediated food allergy with relevant in vitro testing already performed | |
| 6. Suspected non–IgE-mediated food-related reactions | |
| 7. Allergic rhinitis recently investigated in primary care, or referred from a different center for consideration of immunotherapy | |
| 8. Venom allergy recently investigated and referred for consideration of immunotherapy |
ACEi, Angiotensin-converting-enzyme inhibitor; CRSwNP, chronic rhinosinusitis with nasal polyps; HR, hypersensitivity reaction; NSAID, nonsteroidal anti-inflammatory drugs.
Suspected perioperative HR excluded.
TM appears exceptionally well suited for follow-up appointments. The reason for the majority of such appointments was to evaluate the response to treatment for urticaria/angioedema or allergic rhinitis, or assess any accidental exposure to allergen(s) in patients with a new diagnosis of food allergy. Crucially, less than 10% of Nf2f follow-up patients required a further in-person visit for additional allergy testing in agreement with a previous report.7 Therefore, adult allergy centers might want to consider converting all or most f2f follow-up visits to virtual clinics.
In the group of patients referred for suspected drug-induced hypersensitivity, the beta-lactam hypersensitivity was by far the most common reason. A notable 29% of all ADR patients were discharged without requiring further allergy input. These referrals had been previously vetted and considered appropriate for drug allergy assessment based on the information provided by the referring doctor. Nevertheless, the patients who required further testing could be identified only after a careful allergy history had been taken by an allergy specialist. This highlights a potential role for synchronous TM as a triage tool in drug allergy for patient selection before planning further investigations. As previously shown by our team,17 the clinical history in beta-lactam hypersensitivity is a powerful tool with a similar negative predictive value to skin testing in selected cases.
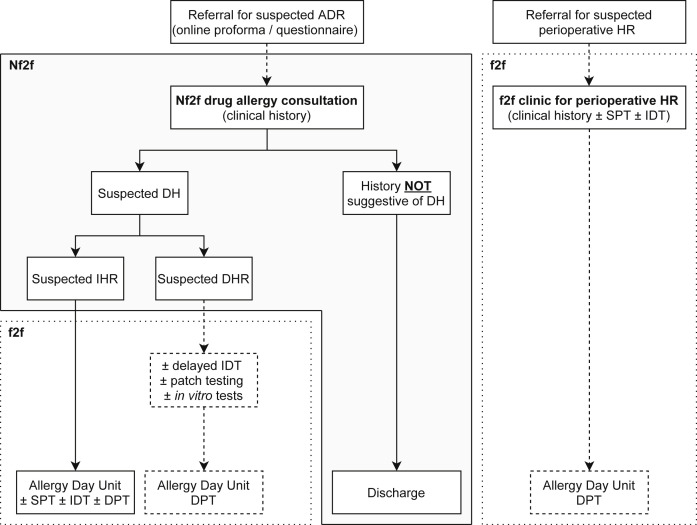
We propose a new drug allergy service model of care for UK adult allergy centers (Figure 2 ). The referring clinician will be prompted to use a standardized questionnaire18 to record the relevant information when referring a patient to a drug allergy service. The questionnaire will be available on the digital platform the primary care physicians are using to refer patients to the allergy service. The referral will be then vetted by the clinicians, and if it is deemed appropriate for drug allergy assessment, an Nf2f appointment will be booked. During the virtual clinic, the allergy specialist will obtain a comprehensive clinical history, and further investigations will be arranged accordingly. As seen in the flowchart, if the history is not suggestive of an ADR, the patient can be discharged from the service with appropriate advice. The investigation of suspected immediate hypersensitivity reactions involves skin testing, if available and validated, and possibly a provocation test to the drug in question.19 These investigations can be carried out in a single in-person visit in the Allergy Day Unit.
Figure 2.
Integrating telemedicine into a drug allergy service model of care. Once the referral for a suspected ADR has been vetted and deemed appropriate for allergy assessment, an Nf2f appointment is booked. Based on the clinical history obtained by the allergy specialist during the Nf2f consultation, an in-person visit can be arranged for further allergy workup, or the patient will be discharged from the service if the history is not suggestive of a hypersensitivity reaction. The separate f2f pathway for suspected perioperative hypersensitivity reactions is also shown. The gray shaded area indicates how telemedicine can be integrated into a drug allergy service model of care. ADR, Adverse drug reaction; DH, drug hypersensitivity; DHR, delayed hypersensitivity reaction; DPT, drug provocation test; f2f, face-to-face; HR, hypersensitivity reaction; IDT, intradermal testing; IHR, immediate hypersensitivity reaction; Nf2f, non–face-to-face; SPT, skin prick testing.
For suspected delayed hypersensitivity reactions, it is not uncommon for more information to be required after the initial allergy assessment, to decide if further tests are indicated. Therefore, Nf2f consultations could be a useful triaging tool for these cases as well, because the process of obtaining additional information, such as copies of the medical notes and drug charts, discharge summaries, and laboratory or biopsy reports, is often a time-consuming administrative challenge. If investigations for nonimmediate drug eruptions are indicated,19, 20, 21 then an in-person visit can be arranged. The pathway for patients referred for suspected perioperative hypersensitivity reactions should be separate and will not be discussed here.
The advantages of TM for both the patient and the clinician are clear.2, 3, 4 , 22 Its integration into the drug allergy service can make it more time- and cost-effective. After an Nf2f assessment, only the clinically relevant drugs will be prepared for skin testing as opposed to all drugs listed in the referral letter. If skin testing is not validated or available, a drug provocation test can be easily arranged after the remote assessment. Finally, given the small number of the UK NHS adult drug allergy centers, this new TM-based model of care could improve access to specialist care, especially for patients who live far from such centers.3
As in other published studies,6 , 7 patients with a working diagnosis of either food or drug allergy were significantly more likely to be recommended further allergy testing and an in-person visit. We acknowledge the fact that each allergist will have his or her own threshold for recommending allergy investigations. Moreover, we found that the more junior members of staff tended to request or recommend more allergy testing.
TM in allergy has been linked with high patient satisfaction.5, 6, 7 Mustafa et al23 collected patient feedback from TM encounters in allergy/immunology during the pandemic. Similar to our findings, they report that 77.4% of the patients felt that their Nf2f appointment was as satisfactory as an in-person encounter. Our overall satisfaction rate was 85%, with 32% of the patients saying that they would have chosen an f2f appointment if they were given the choice. This might reflect the fact that the patients who were under investigation for food or drug allergy often felt that a consultation with a specialist without any testing was incomplete. This stresses the importance of screening criteria for selecting patients suitable for Nf2f appointments to avoid patient disappointment.
Conclusion
TM is an innovative tool that can transform the current models of allergy care. Although the SARS-CoV-2 pandemic is an unprecedentedly unfortunate situation, the unexpected and rapid transition to alternative models of care introduced synchronous TM to the allergy clinicians. If used appropriately, TM can be beneficial for everyone involved. However, standard operating protocols and policies, training, information technology support, quality assurance, and information governance frameworks should all be in place. Large-scale studies of patient outcomes comparing in-person with Nf2f visits are required to identify the best way to integrate TM into clinical practice and assess the long-term effects.
Acknowledgment
The authors acknowledge Zoe Bright for her assistance in obtaining the patient feedback.
I. Thomas had planned the study, obtained the data, performed data analysis, and prepared the manuscript. L.Q.C. Siew was involved in data collection, and preparation and review of the manuscript. K. Rutkowski was involved in the planning of the study as well as the review of the manuscript.
Footnotes
No funding was received for this work.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository
References
- 1.American Telemedicine Association . 2006. Telemedicine, telehealth, and health information technology.https://www.who.int/goe/policies/countries/usa_support_tele.pdf Available from: [Google Scholar]
- 2.Portnoy J.M., Pandya A., Waller M., Elliott T. Telemedicine and emerging technologies for health care in allergy/immunology. J Allergy Clin Immunol. 2020;145:445–454. doi: 10.1016/j.jaci.2019.12.903. [DOI] [PubMed] [Google Scholar]
- 3.Taylor L., Waller M., Portnoy J.M. Telemedicine for allergy services to rural communities. J Allergy Clin Immunol Pract. 2019;7:2554–2559. doi: 10.1016/j.jaip.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Wu A.C., Rehman N., Portnoy J. The good, the bad, and the unknown of telemedicine in asthma and allergy practice. J Allergy Clin Immunol Pract. 2019;7:2580–2582. doi: 10.1016/j.jaip.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Staicu M.L., Holly A.M., Conn K.M., Ramsey A. The use of telemedicine for penicillin allergy skin testing. J Allergy Clin Immunol Pract. 2018;6:2033–2040. doi: 10.1016/j.jaip.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Waibel K.H. Synchronous telehealth for outpatient allergy consultations: a 2-year regional experience. Ann Allergy Asthma Immunol. 2016;116:571–575.e1. doi: 10.1016/j.anai.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Waibel K.H., Bickel R.A., Brown T. Outcomes from a regional synchronous tele-allergy service. J Allergy Clin Immunol Pract. 2019;7:1017–1021. doi: 10.1016/j.jaip.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 8.National Health Service The NHS long term plan. 2019. https://www.longtermplan.nhs.uk/ Available from: Accessed July 15, 2020.
- 9.Fernandez-de-Alba I., Brigido C., Garcia-Gutierrez I., Antolin-Amerigo D., Sanchez-Garcia S. COVID-19 & allergy: allergists workload during the pandemic. [published online ahead of print July 2, 2020]. J Investig Allergol Clin Immunol. [DOI] [PubMed]
- 10.Gonzalez-Perez R., Sanchez-Machin I., Poza-Guedes P., Matheu V., Alava-Cruz C., Mederos Luis E. Pertinence of telehealth in a rush conversion to virtual allergy practice during the COVID-19 outbreak. [published online ahead of print June 8, 2020]. J Investig Allergol Clin Immunol. [DOI] [PubMed]
- 11.Greiwe J. Telemedicine in a post-COVID world: how eConsults can be used to augment an allergy practice. J Allergy Clin Immunol Pract. 2020;8:2142–2143. doi: 10.1016/j.jaip.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Portnoy J., Waller M., Elliott T. Telemedicine in the era of COVID-19. J Allergy Clin Immunol Pract. 2020;8:1489–1491. doi: 10.1016/j.jaip.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuberbier T., Aberer W., Asero R., Abdul Latiff A.H., Baker D., Ballmer-Weber B. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein J.A., Lang D.M., Khan D.A., Craig T., Dreyfus D., Hsieh F. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270–1277. doi: 10.1016/j.jaci.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 15.Krishna M.T., Knibb R.C., Huissoon A.P. Is there a role for telemedicine in adult allergy services? Clin Exp Allergy. 2016;46:668–677. doi: 10.1111/cea.12701. [DOI] [PubMed] [Google Scholar]
- 16.General Medical Council Remote consultations flowchart. https://www.gmc-uk.org/ethical-guidance/ethical-hub/remote-consultations Available from:
- 17.Siew L.Q.C., Li P.H., Watts T.J., Thomas I., Ue K.L., Caballero M.R. Identifying low-risk beta-lactam allergy patients in a UK tertiary centre. J Allergy Clin Immunol Pract. 2019;7:2173–21781.e1. doi: 10.1016/j.jaip.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Brockow K., Ardern-Jones M.R., Mockenhaupt M., Aberer W., Barbaud A., Caubet J.C. EAACI position paper on how to classify cutaneous manifestations of drug hypersensitivity. Allergy. 2019;74:14–27. doi: 10.1111/all.13562. [DOI] [PubMed] [Google Scholar]
- 19.Demoly P., Adkinson N.F., Brockow K., Castells M., Chiriac A.M., Greenberger P.A. International consensus on drug allergy. Allergy. 2014;69:420–437. doi: 10.1111/all.12350. [DOI] [PubMed] [Google Scholar]
- 20.Mayorga C., Celik G., Rouzaire P., Whitaker P., Bonadonna P., Rodrigues-Cernadas J. In vitro tests for drug hypersensitivity reactions: an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2016;71:1103–1134. doi: 10.1111/all.12886. [DOI] [PubMed] [Google Scholar]
- 21.Watts T.J. Investigating nonimmediate drug eruptions: diagnostic benefit of a structured approach. J Allergy Clin Immunol Pract. 2019;7:1324–1326. doi: 10.1016/j.jaip.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Shih J., Portnoy J. Tips for seeing patients via telemedicine. Curr Allergy Asthma Rep. 2018;18:50. doi: 10.1007/s11882-018-0807-5. [DOI] [PubMed] [Google Scholar]
- 23.Mustafa S.S., Yang L., Mortezavi M., Vadamalai K., Ramsey A. Patient satisfaction with telemedicine encounters in an allergy and immunology practice during the coronavirus disease 2019 pandemic. Ann Allergy Asthma Immunol. 2020;125:478–479. doi: 10.1016/j.anai.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]