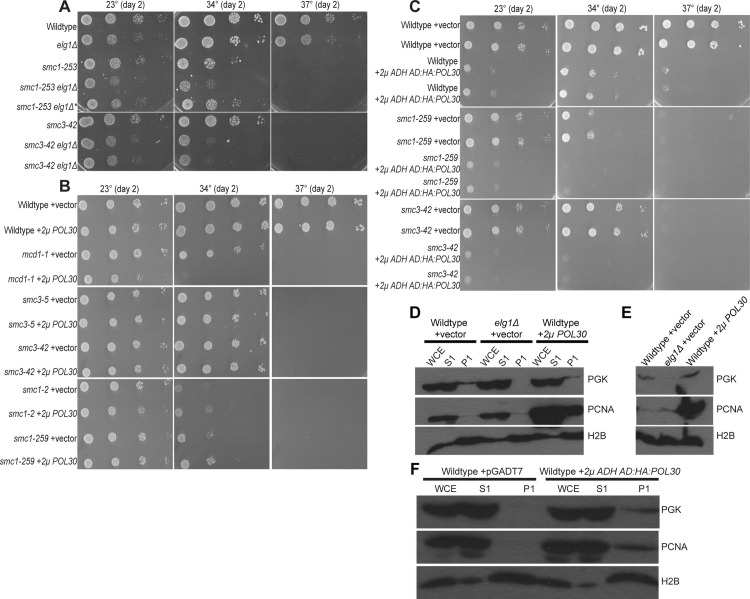
Fig 5. Elevated levels of chromatin-bound PCNA differentially impacts cohesin alleles.
[A] ELG1 deletion exacerbates smc1-259 and smc3-42 mutant cells. 10-fold serial dilution of indicated yeast strains plated on rich medium plates and incubated at 23°C, 34°C, and 37°C for 2 days. * indicates a mutated smc1-259 elg1Δ strain that displays a resistant to temperature sensitivity [B] PCNAOE from a 2μ plasmid specifically impacts mcd1-1 mutant cells. 10-fold serial dilution of indicated yeast strains plated on selective medium plates and incubated at 23°C, 34°C, and 37°C for 2 days. [C] Overexpressed PCNA harboring a GAL4 AD and HA N-terminal tag exacerbates cell growth in wildtype cells, smc1-259, and smc3-42 mutant cells. Images of POL30 in pGADT7 in WT, smc3-42, and smc1-259 mutant cells. 10-fold serial dilution of indicated yeast strains plated on selective medium plates and incubated at 23°C, 34°C, and 37°C for 2 days. [D] Log phase wildtype cells that harbor 2μ POL30 plasmid produce elevated levels of chromatin-bound PCNA, compared to wildtype cells that harbor a vector plasmid and elg1Δ single mutant cells. PGK and H2B were used as a loading control and control for chromatin fractionation, respectively. [E] Five times the chromatin fraction of Fig 5D was loaded to visualize chromatin-bound PCNA in wildtype cells. [F] Overexpressed PCNA via a high copy 2μ plasmid in log phase wildtype cells results in elevated levels of chromatin-bound PCNA compared to log phase wildtype cells harboring a vector plasmid. PCNA was detected by a PCNA specific antibody. PGK and H2B was used as a loading control and control for chromatin fractionation.