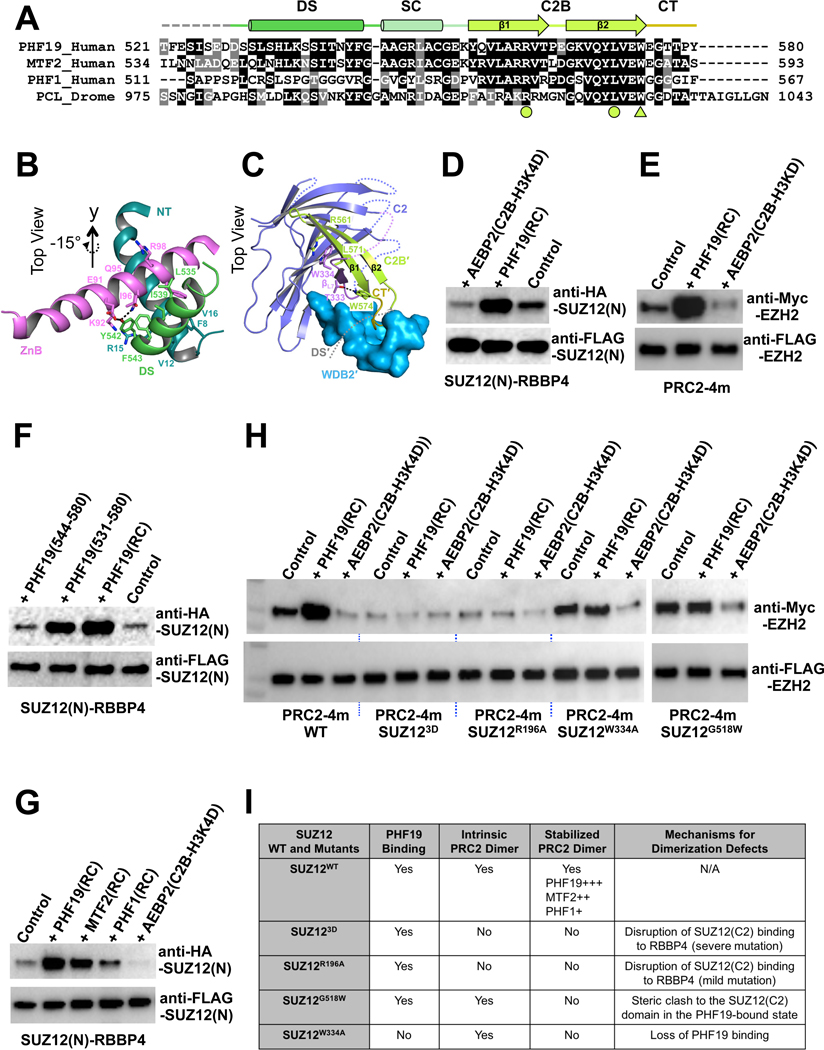
Fig. 3. Stabilization of the intrinsic PRC2 dimer by PHF19.
(A) Sequence alignment of the RC domains of PHF19, MTF2, PHF1 and Drosophila PCL. Functional domains are indicated. The arginine and leucine residues that form the R-W-L triad with residue W334 of the SUZ12(C2) are indicated by filled circles, and the tryptophan residue that forms a hydrogen bond with residue T333 of the SUZ12(C2) is indicated by a filled triangle.
(B) Interactions of the DS helix of PHF19 with the ZnB helix of SUZ12 and the NT helix of RBBP4. Residues mediating hydrophobic and hydrogen bonding interactions are displayed as sticks.
(C) Interactions of the SUZ12(C2), PHF19(C2B) and PHF19(CT) domains. The DS and SC domains of PHF19 are removed from the view for clarity.
(D) Stabilization of the SUZ12(N)-RBBP4 dimer by the PHF19(RC). Co-IP was used to assess the dimer formation. In (D) and (F), equal amounts of the SUZ12(N)-RBBP4 binary complex containing both FLAG-SUZ12(N) and HA-SUZ12(N) were bound to anti-FLAG resin. Anti-FLAG signals served as input control. HA-SUZ12 bound via protein dimerization was assessed by anti-HA antibody. Formation of the intrinsic dimer is indicated by the control lane.
(E) Dimer stabilization and disruption of the four-member PRC2 core complex (PRC2–4m). Different from (D) and (F), equal amounts of PRC2–4m containing both FLAG-EZH2 and Myc-EZH2 were used in (E) and (H). While anti-FLAG signals served as input control, anti-Myc signals indicated the extent of PRC2 dimerization.
(F) Critical role of the DS helix of PHF19 in dimer stabilization. PHF19(RC) corresponding to residues 500–580 was used for crystallization. PHF19 (residues 531–580) is visible in the crystal structure. PHF19(residues 544–580) lacks the DS helix.
(G) Differential dimer stabilization activities of the RC domains of PHF19, MTF2 and PHF1.
(H) Effect of SUZ12 mutations, SUZ123D, SUZ12R196A, SUZ12W334A and SUZ12G518W, on the intrinsic and PHF19-stabilzied dimers of PRC2–4m. Anti-FLAG signals served as input control and anti-Myc signals represented the extent of PRC2 dimerization.
(I) Summary of the structural mechanism of the defect in PRC2 dimerization caused by SUZ12 mutations.
See also Fig. S3.