Figure 2: Timeline of cardiovascular outcome trials with SGLT2 inhibitors.
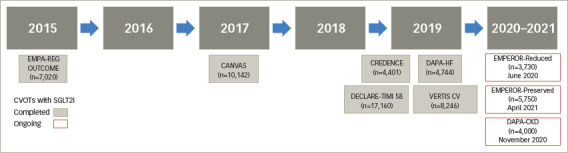
CANVAS = CANagliflozin cardioVascular Assessment Study; CREDENCE = Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation; CVOTs = cardiovascular outcomes trials; DAPA-CKD = Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients with Chronic Kidney Disease; DAPA-HF = Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; DECLARE-TIMI 58 = Dapagliflozin Effect on Cardiovascular Events – Thrombolysis in Myocardial Infarction 58; EMPA-REG Outcome = EMPAgliflozin cardiovascular outcome event trial in type 2 diabetes mellitus patients – Removing Excess Glucose; EMPEROR-Preserved = EMPagliflozin outcomE tRial in patients with chrOnic heaRt failure with preserved ejection fraction; EMPEROR-Reduced = EMPagliflozin outcomE tRial in patients with chrOnic heaRt failure with reduced ejection fraction; SGLT2i = sodium–glucose cotransporter 2 inhibtor; VERTIS CV = eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trials.
