Fig. 3.
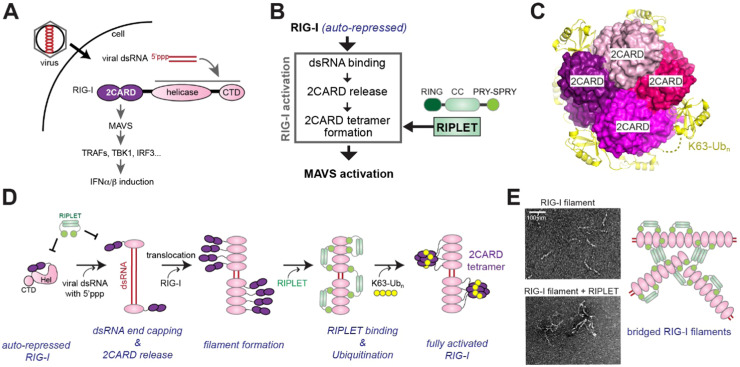
RIPLET is an essential E3 ligase for RIG-I signaling pathway. A. Schematic of RIG-I signaling pathway and domain architecture of RIG-I. During viral infection, viral RNAs accumulate in the cytoplasm. RIG-I detects viral dsRNA with a 5’-triphosphate group (5’ppp), and activates the downstream adaptor molecule MAVS. Activated MAVS in turn induces type I interferon production by triggering TRAF2/3/5/6, TBK1 and IRF3. B. Three steps involved in RIG-I activation. RIG-I binding to dsRNA releases 2CARD auto-repression, but this is not sufficient for signal activation. 2CARD must be tetramerized in order to activate MAVS. RIPLET promotes 2CARD tetramerization by conjugating K63-Ubn to RIG-I, which binds 2CARD and stabilizes its tetramer structure (see C). C. The crystal structure of RIG-I 2CARD (purple-shade colors) in complex with K63-Ubn (yellow) (PDB:4NQK, [54]). K63-Ubn wraps around the 2CARD tetramer, stabilizing its assembly architecture. D. In the absence of viral RNA, RIG-I is in the auto-repressed state. In the presence of viral dsRNA, RIG-I binds dsRNA end recognizing 5’ppp. dsRNA binding triggers ATP hydrolysis, which in turn stimulates translocation along dsRNA, and subsequent recruitment of additional RIG-I molecules. Iterations of the end recruitment and translocation of RIG-I molecules then results in RIG-I filament formation near dsRNA ends. RIPLET binds RIG-I only in the filamentous state, leading to K63-Ubn conjugation, 2CARD tetramerization and MAVS activation. E. RIPLET binding also leads to RIG-I filament bridging and clustering, which results in the amplification of RIG-I signaling. Negative stain electron micrographs are taken from [31].