Fig. 4.
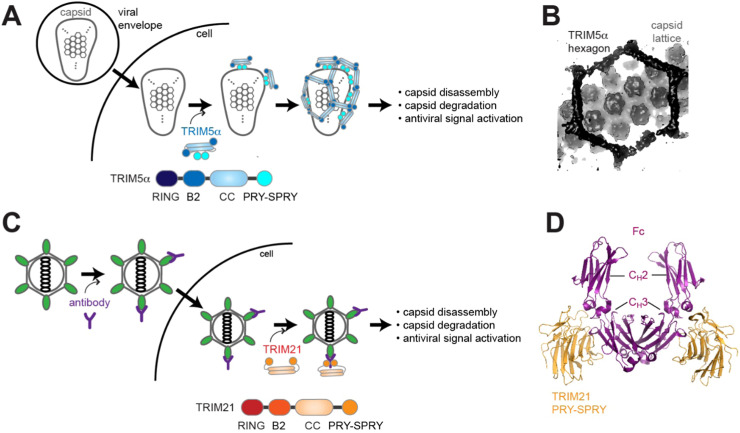
TRIM5α and TRIM21 have dual functions as innate immune receptors and effectors. A. Schematic of TRIM5α function. TRIM5α binds incoming retroviral capsid in a sequence-specific and avidity-dependent manner. Capsid then serves as a template to further assemble hexagonal lattice of TRIM5α. This results in both the effector function of capsid disassembly and degradation as well as the receptor function of antiviral signal activation. Ubiquitination activity of TRIM5α is thought to mediate both of these functions, although some studies proposed a direct role of TRIM5α as an autophagy receptor [125], [126]. B. Cryo-electron microscope reconstruction map of TRIM5α hexagonal lattice bound to HIV-1 capsid lattice (EMD-20565, [32]). C. Schematic of TRIM21 function. Microbial pathogen partially coated with antibody may evade neutralization, and enter the cytosolic compartment. TRIM21 recognizes the Fc portion of the antibody, which triggers the auto-ubiquitination activity and subsequently its antiviral functions similar to those of TRIM5α.(D.) Crystal structure of TRIM21 PRY-SPRY in complex with a dimeric Fc portion of IgG (PDB:2IWG, [39]).