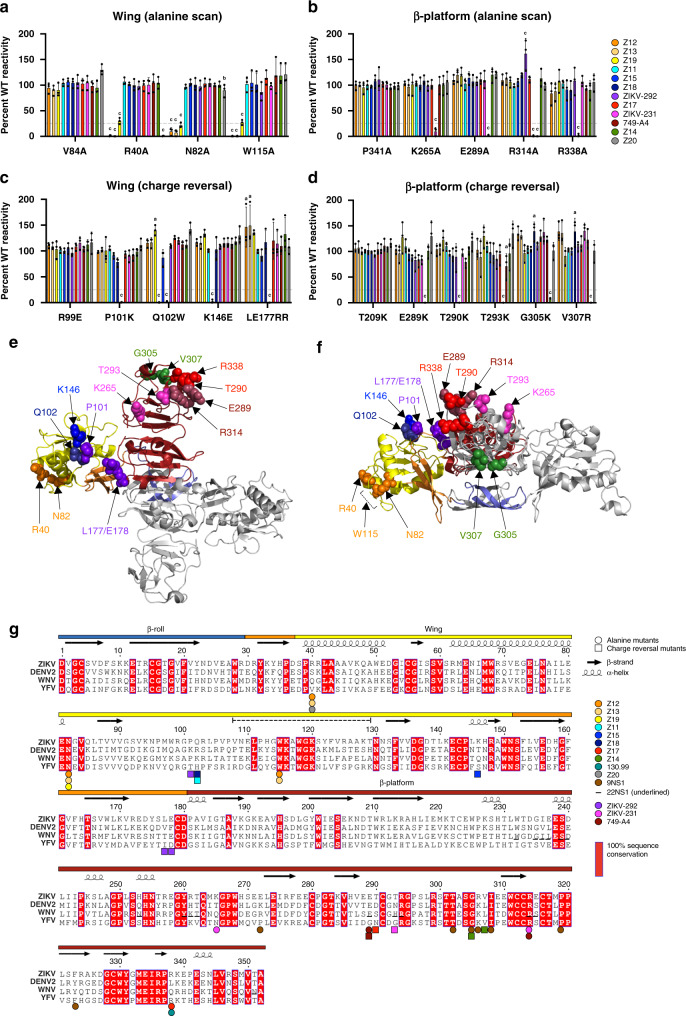
Fig. 4. Epitope mapping of anti-ZIKV NS1 mAbs.
Critical binding residues were mapped using a, b an alanine-scanning library and c, d targeted charge-reversal mutants. 293T cells were transfected with plasmids encoding ZIKV NS1 and the mAb reactivity to each mutant relative to WT NS1 was measured by flow cytometry. For each mutant, the relative mAb reactivity was normalized to the relative reactivity of an oligoclonal staining cocktail. Critical residues were defined as those mutants with <25% binding compared to WT NS1. Data are shown for critical residues in the wing (a, c) and β-platform (b, d) domains. A minimum of 5000 cells was collected for each sample. Data are the average of three experiments (error bars represent the mean ± SD) and were analyzed by two-way ANOVA with Holm–Sidak’s multiple comparison of each mutant to a V84A, b P341A, c R99E, or d T209K. Superscript letters indicate significance: a, p < 0.01; b, p < 0.001; c, p < 0.0001 (Z12: LE177/178RR, p = 0.009; Z13: LE177/178RR, p = 0.002; Z18: G305K, p = 0.007; V307R, p = 0.007; Z19: Q102W, p = 0.007; Z20: N82A, p = 0.0002; 749-A4: T293K, p = 0.0068). e, f Mapping of critical residues for anti-NS1 mAbs onto the crystal structure of the ZIKV NS1 dimer (PDB 5K6K) in top view (e) or side view (f). Critical residues are indicated by spheres and are color-coded according to mAb panel in a–d. In each structure, one monomer is gray and one is color-coded by domain (blue, β-roll; yellow, wing; red, β-platform; orange, connector subdomain and greasy finger). This figure was prepared using PyMOL. g Secondary structure representation of epitopes in ZIKV NS1 as defined by alanine-scanning (circles) and charge-reversal mutagenesis (squares). Sequence alignment is shown for ZIKV (H/PF/2013), DENV2 (Thailand/16681/84), WNV (NY99), and YFV (17D); red shading indicates 100% sequence conservation. Colored bars above the alignment indicate domains: β-roll (blue, 1–29); wing (yellow, 30–180) with connector subdomain (orange); β-platform (red, 181–352). The dashed line above the alignment indicates the wing flexible loop (108–129). The corresponding contact residues of 22NS1, an anti-WNV NS1 mAb, which were determined by X-ray crystallography30, are shown underlined in the alignment for comparison. This figure was prepared using ESPript 3.079. Source data are provided as a Source Data file for a–d.