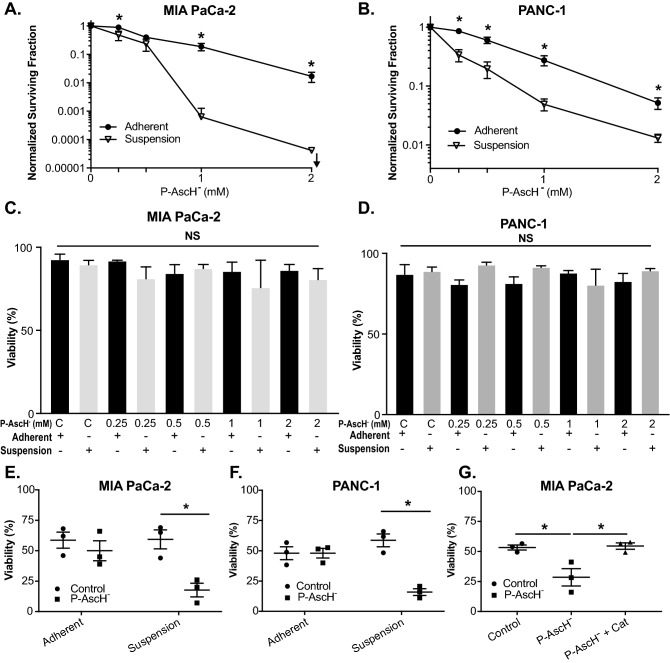
Figure 3.
P-AscH− induced susceptibility to non-adherent PDAC cells is mediated by hydrogen peroxide. PDAC cells were treated with varying doses (0–2 mM) of ascorbate for 1 h under adherent or suspension conditions. Following P-AscH− treatment, cells were plated for a clonogenic cell survival assay and assessed for viability. (A) MIA PaCa-2 or (B) PANC-1 cells treated with P-AscH− in suspension conditions had decreased clonogenic survival compared to PDAC cells treated in adherent conditions. Data represent normalized surviving fractions compared to controls ± SE (n = 4, *p < 0.05; Two-tailed unpaired Student’s t test comparing adherent vs. suspension results at each P-AscH− concentration). (C) MIA PaCa-2 or (D) PANC-1 cells treated in suspension conditions with P-AscH− had no changes in viability compared to PDAC cells treated in adherent conditions. Data represent percent viability ± SE (n = 4, p > 0.05; one-way ANOVA with Tukey’s multiple comparisons). PDAC cells were treated with ascorbate (1–2 mM) for 1 h under adherent or suspension conditions. Following P-AscH− treatment, cells were subjected to fluid shear stress (FSS) and assessed for viability. (E) MIA PaCa-2 and (F) PANC-1 cells treated in suspension conditions were more susceptible to P-AscH− treatment compared to PDAC cells treated in adherent conditions when assessed during FSSs. Data represent percent viability compared to controls ± SE (n = 3, *p < 0.05; two-way ANOVA with Bonferroni’s multiple comparisons). (G) Catalase reverses ascorbate-induced cytotoxicity during FSS. MIA PaCa-2 cells treated in suspension conditions with P-AscH− were susceptible to FSS and this effect was mitigated by the addition of catalase (200 U/mL). Data represent percent viability compared to controls ± SE (n = 3, *p < 0.05; one-way ANOVA with Tukey’s multiple comparisons).