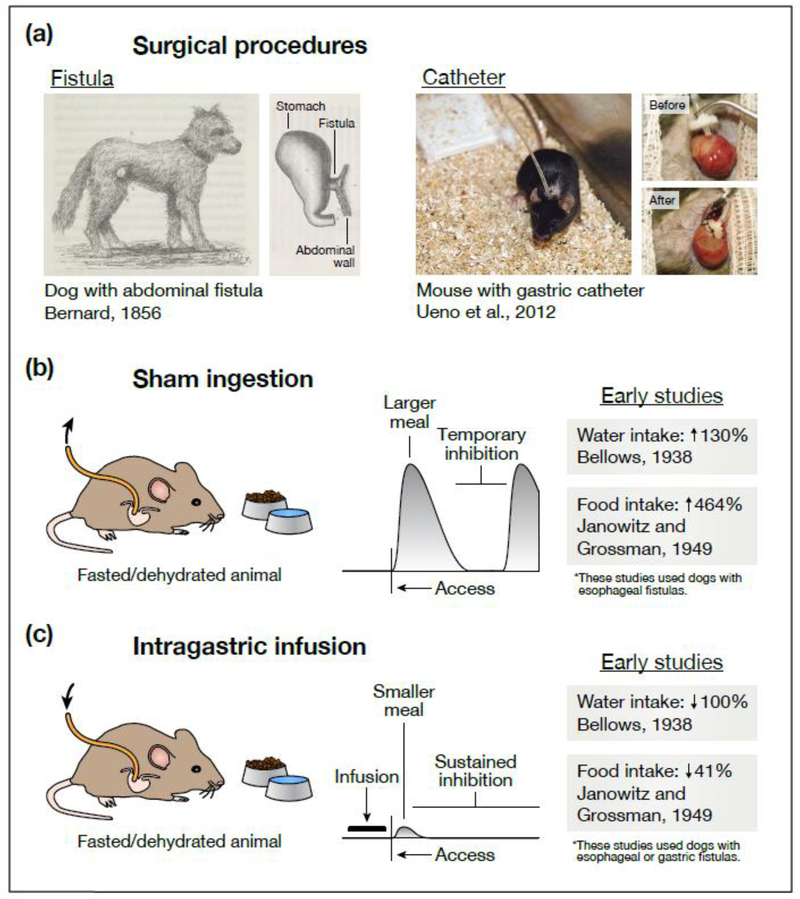
Figure 1. Effects of gastrointestinal manipulations on eating and drinking behavior.
(a) Two experimental techniques—sham ingestion and intragastric infusion—allow investigators to manipulate the contents of the gastrointestinal tract during eating and drinking. Today, both techniques frequently rely on a surgical procedure called catheterization, in which one end of a catheter is permanently attached to an animal’s stomach while the other end remains outside the body. Earlier experiments—often employing dogs or other large animals—used a similar procedure called fistulization, in which a portion of the esophagus, stomach, or intestines is ruptured and surgically attached to the animal’s exterior (either directly or via a steel cannula) so that tubing can be temporarily attached during experiments. (b) During sham feeding or drinking, the external end of the catheter is left open so that any ingested food or water drains from the animal before reaching the stomach. This has two striking effects. First, animals consume much larger meals than they would during normal ingestion. Second, while animals will eventually pause eating or drinking, this inhibition is only transient. These observations suggest that pre-gastric signals alone can produce temporary (but not persistent) satiation. (c) During intragastric infusion, the external end of the catheter is used to infuse food or water directly into the stomach, bypassing the mouth and throat altogether. Infusions into hungry or thirsty animals dramatically (sometimes completely) reduce intake when food or water is subsequently presented. This suggests that post-ingestive signals are sufficient to durably satiate hunger and thirst. However, the exact nature of these signals (what sensory information they convey, where in the gut they are generated) cannot be determined from behavioral observations alone. In (b), the listed water intake is the volume sham drunk (fistula open) relative to the volume drunk normally (fistula closed) [2], and the listed food intake is the time spent sham feeding (after fistulization) relative to the time spent feeding normally (before fistulization) [5]. In (c), the listed water intake is the volume sham drunk after intragastric infusion (water was presented 20 min after the dog’s entire fluid deficit was infused; the dog refused to drink) relative to the volume sham drunk without prior infusion [2], and the listed food intake is the amount eaten normally after intragastric infusion (food was presented 20 min after ∼43% of the dogs’ normal meal size was infused) relative to the amount eaten without prior infusion [5]. In the schematics in (b) and (c), the horizontal axis represents time and the vertical axis represents rate of ingestion. These schematics are based on many of the references cited, although it is important to note that they are generalizations and that observations have varied across studies and species. We direct the reader to Chapters 2–4 of ref. [78] and Chapter 19 of ref. [79] for a more thorough discussion. The illustrations in (a) are adapted with permission from Figures 27 and 56 of ref. [4] (public domain, https://gallica.bnf.fr/ark:/12148/bpt6k6214318x) and from Figures 2 and 3 of ref. [80] (copyright 2012, Springer Nature).