Figure 1. Developmental regulation of SNPH-mediated anchoring of axonal mitochondria.
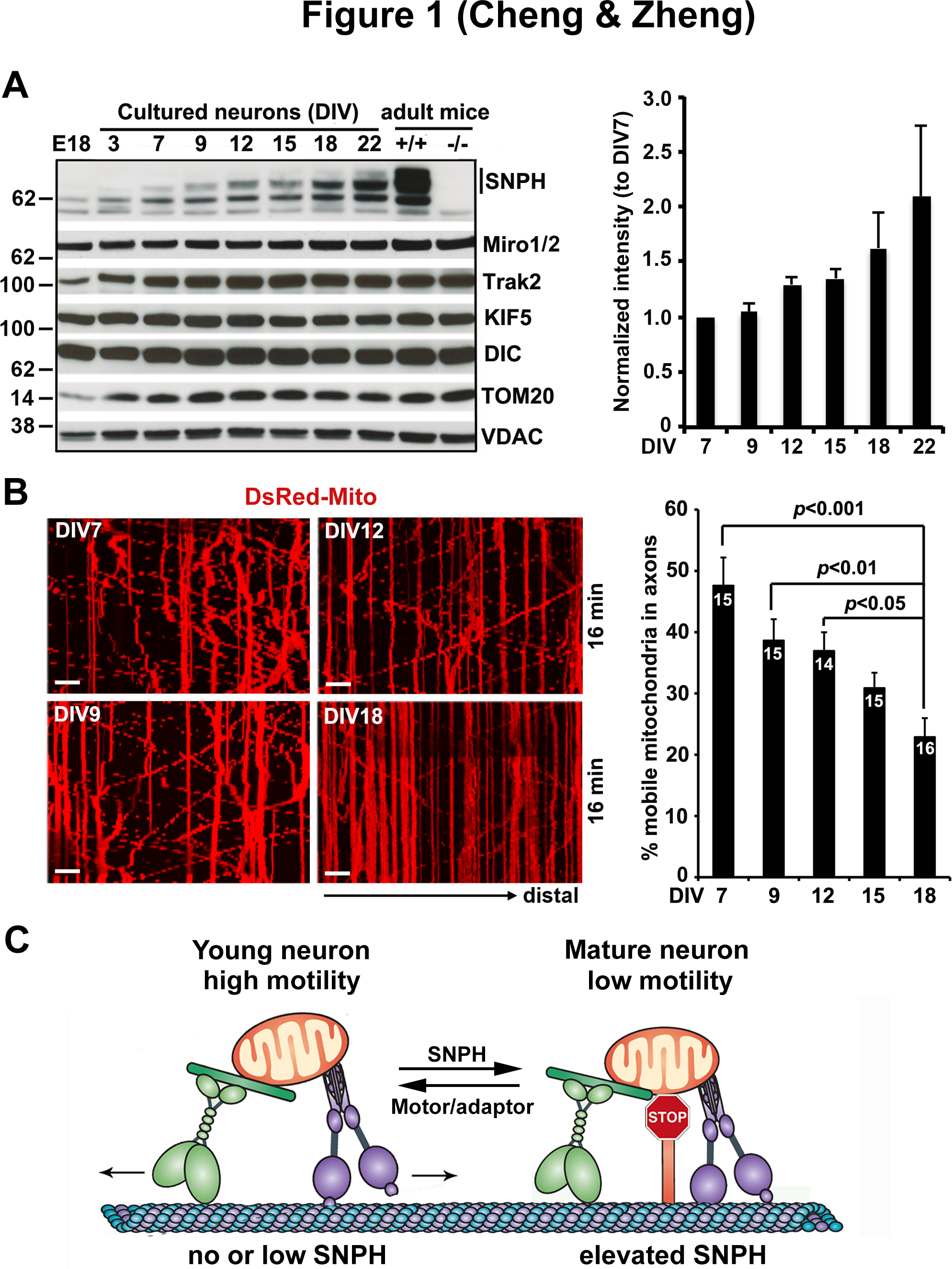
(A) Representative immunoblots and quantitative analysis showing a progressive increase in SNPH expression with neuronal development and maturation. Note that while SNPH expression is abundant in adult mouse brains, it is hardly detectable in E18 brains and in cultured neurons before DIV3, becomes readily detectable in cultured neurons after DIV9, and peaks at DIV22. This expression pattern is specific to SNPH and does not apply to mitochondrial motor-adaptor proteins (KIF5, DIC, Miro1/2, and Trak2) or outer mitochondrial membrane proteins (TOM20 and VDAC).
(B) Representative kymographs and quantification from time-lapse live imaging of mouse cortical neuron axons showing a progressive decline of mitochondrial motility with maturation from DIV7 to DIV18. In kymographs, vertical lines represent stationary mitochondria; oblique lines or curves to the right indicate anterograde transport toward distal terminals, to the left denote retrograde transport toward the cell body. Scale bars:10 μm.
(C) Model illustration of mechanistic link between mitochondria motility and SNPH expression with neuron maturation. In young neurons where SNPH expression is not detectable or extremely low, axonal mitochondria display robust motility; whereas in mature neurons with high SNPH expression, the majority of axonal mitochondria remain stationary.
Data in (A) and (B) are adapted from Zhou, B., Yu, P., Lin, M.Y., Sun, T., Chen, Y., Sheng, Z.H., 2016. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. The Journal of Cell Biology 214, 103–119.
