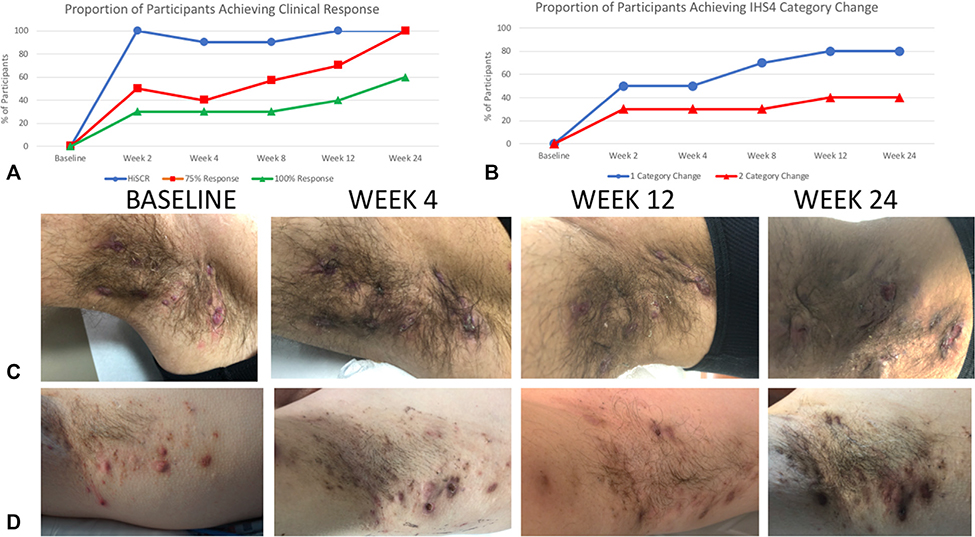
Fig 1.
Measures of clinical response to brodalumab therapy in hidradenitis suppurativa. A, The proportion of patients achieving HiSCR at weeks 12 and 24 was 100%, with 75% reduction in AN counts (red) and 100% reduction in AN counts (green) in 60% and 40% of participants, respectively. B, Measurement of clinical outcomes using IHS4 category change shows 80% and 40% of patients achieving 1-category and 2-category change respectively. C and D, Representative clinical photos show a rapid reduction in the inflammatory nature of nodules at week 4 compared to baseline and continued improvement at week 12. HiSCR was maintained at Week 24 despite external triggers initiating limited flares of disease. AN, Abscess and nodule count; HiSCR, Hidradenitis Suppurativa Clinical Response; IHS4, International Hidradenitis Suppurativa Severity Score.