Abstract
The complement system is an ancient arm of the innate immune system that plays important roles in pathogen recognition and elimination. Upon activation by microbes, complement opsonizes bacterial surfaces, recruits professional phagocytes, and causes bacteriolysis. Borreliella species are spirochetal bacteria that are transmitted to vertebrate hosts via infected Ixodes ticks and are the etiologic agent of Lyme disease. Pathogens that traffic in blood and other body fluids, like Borreliella, have evolved means to evade complement. Lyme disease spirochetes interfere with complement by producing a small arsenal of outer surface lipoproteins that bind host complement components and manipulate their native activities. Here we review the current landscape of complement evasion by Lyme disease spirochetes and provide an update on recent discoveries.
Keywords: complement evasion, Lyme disease, pathogenesis
Lyme disease
Lyme disease is a significant public health burden and remains the most prevalent vectorborne illness in the United States with diagnosed cases estimated between 224,000 to 444,000 per year (1-3). Worldwide, Lyme disease is caused by Borreliella spirochete infections, including B. burgdorferi, B. afzelii, and B. garinii, as well as other Borreliella species (4, 5). Lyme disease spirochetes are transmitted to humans by the bite of infected ticks of the Ixodes genus. During the infectious process, Borreliella spirochetes disseminate to distant organs including joints, heart, and the central nervous system (6-8). If left untreated, Lyme borreliosis causes a wide range of symptoms with early stages of infection characterized by general malaise, fever, fatigue, and aches in joints, along with 70 to 80 percent of patients presenting with an erythema migrans rash. Early infection is localized near the site of the tick-bite and quickly disseminates throughout the infected host, where it can colonize deeper tissues (9). Following dissemination, and in the absence of treatment, additional symptoms of Lyme disease can include neurological sequelae and carditis (6). In some instances, arthritis with severe joint pain can ensue that is difficult to treat. Lyme disease spirochetes can persist in immunocompetent hosts and, given their extracellular nature, must possess mechanisms to evade host immunity including the complement system.
The complement system
The complement system serves as a first-line-of-defense against invading microorganisms (10, 11). Among the many proteins that comprise the complement system are pattern recognition molecules (e.g. C1q, mannose-binding lectin (MBL), ficolins, collectins), serine proteases (e.g. C1r, C1s, factor B, factor D, etc.), circulating precursor components (e.g. C3, C4, C5, etc.) and endogenous regulator proteins (discussed below). Complement is activated through three canonical pathways known as the classical pathway (CP), lectin pathway (LP), and alternative pathway (AP). Binding of surfaces by complement pattern recognition molecules initiate complement activation by the CP and LP, whereas the AP is continuously activated at low levels. Regardless of the triggering event, all pathways coalesce at the central molecule of the cascade, complement component C3. C3 is converted to C3a and C3b by enzymatic protein complexes known as C3 convertases (i.e. C4b2b and C3bBb) releasing C3a into the milieu and resulting in covalent attachment of C3b to the activating surface. At high C3b surface densities, C3 convertases bind additional C3b molecules and switch substrate specificities to become C5 convertases (i.e. C3bBbC3b and C4b2bC3b). Cleavage of C5 releases the powerful anaphylatoxin C5a while C5b interacts with complement components C6, C7, C8, and C9 to form a lytic pore structure known as the membrane attack complex (MAC) (i.e. C5b-9). The activation of complement is tightly controlled by proteins known as regulators of complement activation (RCAs) which protect host tissues from inappropriate complement targeting (12). Notable RCAs include C1 esterase inhibitor, factor H (FH), C4b-binding protein (C4BP), decay accelerating factor, CD59, membrane cofactor protein, and complement receptor 1.
Complement activation by Lyme disease spirochetes
Lyme disease spirochetes encounter complement during the tick bloodmeal, throughout hematogenous dissemination, and within host tissues distant from the site of the tick bite. If a spirochete is unable to evade detection by one of the three complement pathways, initiating serine proteases begin converting zymogen complement proteins into activated protein fragments resulting in several synergistic host defense mechanisms that include: i) opsonization (C1q, C3b, C4b); ii) phagocyte recruitment (C3a, C5a); iii) priming of the adaptive immune system (C1q, C3b, C4b, C3a, C5a); and iv) bacteriolysis (MAC/C5b-9) (Key Figure; Figure 1). Because serum-mediated bacteriolysis is a byproduct of MAC formation, the relative resistance of Lyme disease spirochetes to killing by serum has been used to assess the ability of various Borreliella species to block complement activation (13, 14). An emerging theme is that Borreliella species, as well as strains within a given species, may vary widely in their susceptibility to direct killing by human complement, and more generally, complement resistance by Lyme disease spirochetes varies across different vertebrate hosts. While it is worth noting that certain Borreliella strains do not follow their species-level categorization, B. burgdorferi and B. afzelii are generally characterized as serum resistant, whereas B. garinii is characterized as more susceptible to killing by human serum.
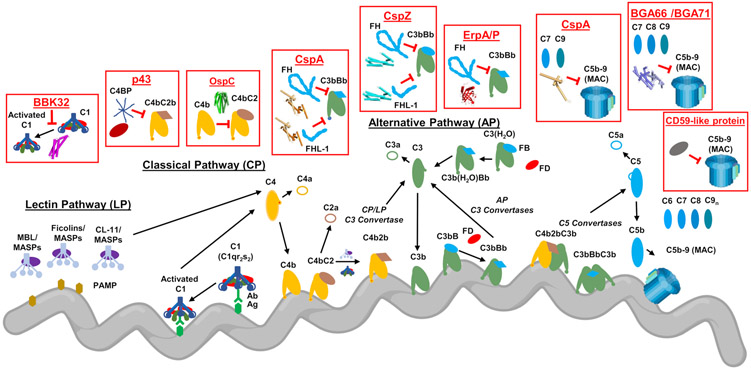
Figure 1. Complement Evasion by Lyme Disease Spirochetes.
To evade complement, Lyme disease spirochetes produce outer surface lipoproteins that bind directly to complement components. These proteins either inhibit complement activity directly or bind to host-derived regulators of complement activity (RCA) and thereby attenuate complement activation at the spirochete surface. Several of these inhibitors block the upstream initiation steps of the cascade including the CP-specific inhibitor BBK32, which binds to C1r within the C1 complex and traps C1 in a zymogen state. OspC binds C4b and interferes with the activation of both the CP and LP by preventing formation of the CP/LP C3 proconvertase (i.e. C4bC2). Lyme disease spirochetes also produce an outer surface protein of unknown identity termed p43 that downregulates the CP and LP by recruiting the primary RCA of these two pathways called C4b-binding protein (C4BP). CspA is among a group of three structurally unrelated proteins that bind the dominant negative regulator of the AP known as factor H (FH). CspA also binds to factor H-like protein 1 (FHL-1), a molecule of the FH family that retains complement regulatory activities. CspZ, a second FH/FHL-1-binding protein, also downregulates the formation of AP C3 and C5 convertases on the spirochete surface. A third type of FH-binding protein are the paralogs ErpA/ErpP/ErpC. Among these, ErpA and ErpP bind to FH (but not to FHL-1). Borreliella produce at least four proteins that block the formation of the MAC complex. CspA binds C7 and C9 in a FH-independent manner and blocks C9 polymerization. Two homologous proteins from B. bavariensis, BGA66 and BGA71 also block C9 polymerization by binding to C7, C8, and C9. Finally, an unidentified outer surface protein is produced by Lyme disease spirochetes that exhibits similar activity to the RCA known as CD59. PAMP: pathogen-associated molecular pattern; Ab: antibody; Ag: antigen; CL-11: collectin-11. This figure was created using BioRender.com.
Mechanisms of complement evasion by Lyme disease spirochetes
The ability of Borreliella species (and strains therein) to resist serum-mediated killing suggests they possess underlying complement evasion mechanisms. Lyme disease spirochetes produce a robust complement evasion repertoire in the form of outer surface lipoproteins that harbor specific anti-complement activities. To date, nearly a dozen such proteins have been identified (Figure 1 and Table 1). The activity of each of these proteins fall into two general classifications: i) those that recruit functional host RCAs to the spirochete surface resulting in downregulation of complement and ii) borrelial outer surface proteins that bind directly to a specific complement protein and interfere with its native function. The temporal and spatial regulation of gene expression and relative roles of borrelial complement evasion proteins in experimental Lyme borreliosis are the subject of several excellent reviews (15-23). Here, we will focus on the distinct nature of each of these proteins including their three-dimensional structures, their molecular interactions with host proteins, and recent studies related to their functions in complement evasion.
Table 1.
Borreliella Species Lipoproteins Involved in Complement Evasion
| Protein/Factor | Species | Mechanism | Target | Gene | PDB# |
|---|---|---|---|---|---|
| BBK32 | burgdorferi, afzelii | Direct | C1r, C1 | bbk32 | 6N1L |
| BGA66 | bavariensis | Direct | C7, C8, C9 (C5b-9) |
bga66 | NA |
| BGA71 | bavariensis | Direct | C7, C8, C9 (C5b-9) |
bga71 | 6FMH, 6FL0 |
|
CD59-like protein |
burgdorferi | Direct | (C5b-9) | unknown | N/A* |
| CspA (CRASP-1) | burgdorferi, afzelii, spielmanii | RCA recruitment, Direct | FH, FHL-1, C7, C9, (C5b-9) | cspA/bba68 | 1W3Z, 1W33, 4BL4, 5A2U |
| CspZ (CRASP-2) | burgdorferi | RCA recruitment | FH, FHL-1 | cspZ/bbh06 | 4BG0, 4CBE |
| ErpA (CRASP-5) | burgdorferi | RCA recruitment | FH, FHR-1, FHR-2, FHR-5, | erpA/bbp38/bbl39 | 5NBQ, 4J38, 2M4F |
| ErpC (CRASP-4) | burgdorferi | RCA recruitment | FHR-1, FHR-2 | erpC | 4BF3, 4BOD, 4BXM |
| ErpP (CRASP-3) | burgdorferi | RCA recruitment | FH, FHR-1, FHR-2, FHR-5 | erpP/bbn38 | 4BOB |
| OspC | burgdorferi | Direct | C4b | ospC/bb_b19 | 1GGQ, 1F1M, 1G5Z |
| p43 | burgdorferi | RCA recruitment | C4BP | unknown | N/A* |
N/A = Not applicable
Recruitment of RCAs to the Lyme disease spirochete surface
Previously, a B. burgdorferi, B. garinii, and B. afzelii surface protein that binds C4BP, termed p43, was identified, as was a B. burgdorferi CD59-like protein; however, the borrelial genes encoding these proteins are not known (24, 25). The predominant RCA that is recognized by Lyme disease spirochetes is the major negative regulator of the AP, known as FH (26-29). FH is a 155 kDa glycoprotein composed of twenty sequentially arranged complement control protein (CCP) domains (28). FH acts by binding to C3b and competing with factor B for proconvertase formation (i.e. C3bB), as well as by stimulating the nonreversible release of the Bb fragment in previously formed C3bBb convertases (i.e. decay accelerating activity). FH also acts as a cofactor for the factor-I mediated degradation of C3b. Lyme disease spirochetes produce three different types of FH-binding proteins that are collectively known as complement regulator-acquiring surface proteins (CRASPs). Among the CRASPs are CspA (also known as CRASP-1, BBA68, or FHBP), CspZ (also known as CRASP-2 or BBH06) and three paralogs of the OspEF-related protein (Erp) family, ErpA (also known as OspE, CRASP-5, or BBP38, among others), ErpC (CRASP-4), and ErpP (CRASP-3). Hereafter these proteins will be referred to by CspA, CspZ, ErpA, ErpC, and ErpP, respectively (see (17, 23) for a comprehensive review of Borreliella FH-binding protein nomenclature).
CspA is a ~25 kDa surface-localized lipoprotein encoded on the linear plasmid lp54 (30, 31). The loss of B. burgdorferi cspA renders borrelial cells sensitive to human serum (31, 32). Furthermore, heterologous surface production of B. burgdorferi CspA protects a previously serum sensitive B. garinii strain from complement-mediated killing (33). CspA interacts with human FH with high-affinity, exhibiting an equilibrium dissociation constant (KD) of 28 nM (31). Correlating to the relative serum sensitivity of each genospecies, homologs of CspA in B. afzelii retain high-affinity binding of human FH, whereas B. garinii homologs do not (34). Crystal structures of CspA have revealed a novel helical fold whereby intermolecular contacts from the C-terminal alpha-helix mediate formation of a CspA homodimer (Figure 2) (35, 36). Importantly, disruption of the CspA dimer by mutagenesis causes complete loss of FH-binding activity (37). Among the eleven members of the paralogous gene family known as Pfam54, only CspA is capable of binding to FH (38). It has been hypothesized that differences in the amino acid sequences at the C-termini of non-CspA Pfam54 proteins prevents formation of ‘CspA-like’ homodimers and therefore abrogates FH-binding activity (37, 39). In support of this idea, a recent crystal structure of the Pfam54 member BBA69 revealed that, although the CspA-like helical fold is conserved, BBA69 – which does not bind FH – lacks an extended C-terminal helix and thereby forms only monomers (40).
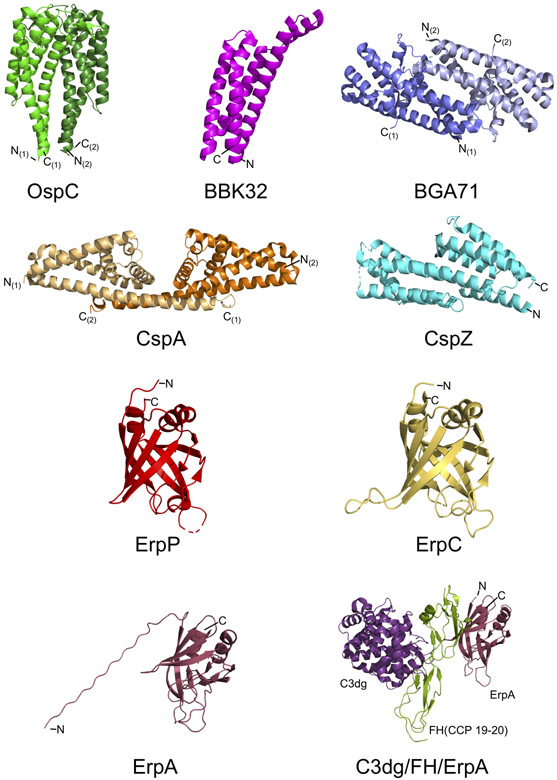
Figure 2. Three-dimensional structures of proteins involved in Lyme disease spirochete complement evasion.
High resolution crystal structures have been solved for many of the known Lyme disease spirochete complement evasion proteins. Shown above are the crystal structures of unbound OspC (PDB#: 1GGQ), BBK32 (PDB#: 6N1L), BGA71 (PDB#: 6FMH), CspA (PDB#: 1W33), CspZ (PDB#: 4CBE), ErpP (PDB#: 4BOB), and ErpC (PDB#: 4BXM). An NMR solution structure of ErpA has also been solved (PDB#: 2M4F; BMRB#: 19001). Co-crystal structures have also been solved for ErpA in complex with FH CCP19-20 (PDB#: 4J38; not shown) and a ternary complex of ErpA/FH CCP19-20/C3dg (PDB#: 5NBQ). N- and C- termini are labeled. For dimeric structures, each monomer is denoted by parentheses. Images were prepared from the deposited Protein Data Bank structure coordinates using PyMOL (The PyMOL Molecular Graphics System, Version 2.0 Schrodinger, LLC).
CspZ is a second outer surface lipoprotein produced by Lyme disease spirochetes that functionally recruits human FH to the bacterial surface. CspZ is a ~23kDa protein encoded by the cspZ gene located on the linear plasmid lp28-3 of B. burgdorferi strain B31 (41, 42). CspZ shares no significant sequence homology to CspA nor to other genes within the B. burgdorferi genome. Like cspA, heterologous expression of cspZ can protect serum sensitive spirochetal strains (42). The three-dimensional structure of CspZ is characterized by a single domain alphahelical protein (Figure 2) (43). Unlike CspA, CspZ is predicted to bind FH with a monomeric binding site (43).
A third type of FH-binding protein from Lyme disease spirochetes are a group of three related proteins known as ErpA, ErpC, and ErpP. Surface plasmon resonance (SPR) experiments showed that while B. burgdorferi ErpA bound strongly to human FH, ErpP bound weakly and ErpC not at all (44). Recently, high resolution three-dimensional structures of each protein have become available including a co-crystal structure of ErpA from B. burgdorferi strain N40 in a ternary complex with FH and C3dg (Figure 2) (45-47). These structures, along with the unbound structures of ErpP and ErpC, implicate a loop insertion within ErpC that may prevent it from binding to human FH (45, 47). Unlike CspA and CspZ, when ErpA, ErpP, or ErpC are produced heterologously in a serum sensitive B. garinii strain they fail to protect the spirochetes from human complement (48, 49). However, overexpression of ErpA protected a serum sensitive B. burgdorferi cspA mutant from human serum (50). These results have called into question whether the FH-binding activities of these proteins are of greater potential relevance to the FHrelated proteins (FHRs) and/or FH-like proteins (FHLs) that are discussed below.
The AP regulatory activities of FH arise from two primary C3b-binding sites involving FH-CCPs 1-4 and FH-CCPs 19-20 (26, 29, 51). However, to act on surface-bound C3b, soluble FH attaches to host cells via two independent sialic acid binding sites located on FH-CCP7 and FH-CCP20 (52). Like host cells, Borreliella must recruit FH to the cell surface in a functionally active form. To mimic the physiological orientation of active FH on bacterial cells, it has been proposed that microbes target FH at common sites overlapping the FH/sialic acid binding sites (53). Indeed, Lyme disease spirochetes have adopted this strategy, as CspA and CspZ have both been shown to bind native FH primarily via a site on CCP7, while ErpA binds FH preferentially at FH-CCP20 (30, 47, 54). Furthermore, the ErpA binding site on FH-CCP20 overlaps the known FH-CCP20 sialic acid binding site as judged by the co-crystal structures of ErpA/FH(CCP 19-20)/C3dg and sialic acid/FH(CCP 19-20)/C3d (47, 55).
While many microbes are known to produce FH-binding proteins (56, 57), it is interesting that Lyme disease spirochetes retain an apparently functionally overlapping set. One potential explanation are differences in temporal production as has been shown for CspA and CspZ (16, 58-61). This suggests that the production of CspA and CspZ are important at unique stages of the enzootic cycle. Another possibility, which is not mutually exclusive, may be related to differences in how each CRASP protein interacts with other members of the FH protein family called FHL and FHR proteins. Human FH is part of a larger family of proteins that include FH molecules of various sizes and differing functions (62). For example, FHL-1 is an alternative splice variant of FH that consists of FH CCP1-7 and, although it has different cell surface specificity than FH, it retains the decay accelerating activity and co-factor activity of the full-length molecule (62). Furthermore, FHL-1 has been proposed to be the main complement regulatory protein over FH on certain host cell types (62). Consistent with domain mapping on FH (i.e. CCP7), CspA and CspZ bind to FHL-1 whereas ErpA, ErpP, or ErpC do not. Instead, the Erp proteins have been shown to bind to the FHR proteins FHR-2, FHR-3, and FHR-5, which each contain domains highly homologous to FH-CCP19-20 (49). While the interaction of CspA and CspZ with FHL-1 would be expected to contribute to complement evasion, the role of FHR binding by ErpA, ErpP, or ErpC is less clear. This is because, although the function of FHRs in complement regulation is an area of active research, there are several lines of evidence supporting their role in regulating the activities of FH and FHL-1 rather than regulating complement activation directly (62). Furthermore, complement-independent roles for some FHR proteins have also been described (63).
Direct inhibition of complement by Borreliella outer surface proteins
While the borrelial proteins discussed above rely on commandeering the native function of endogenous complement regulators, Lyme disease spirochetes also produce outer surface proteins that bind directly to complement proteins and inhibit their function. These include three proteins that block downstream MAC formation (BGA66, BGA71, and CspA) and two proteins that block the upstream initiation steps of complement (OspC and BBK32).
In addition to binding FH, CspA can directly block MAC formation in a FH-independent manner (64). The mechanism for this activity arises from the ability of CspA to bind directly to C7 and C9 with moderate affinities (KD’s = 5.1 and 3.4 μM, respectively) and inhibit C9 polymerization (64). This study also showed that C7 and C9 do not bind to the same site as FH on CspA nor do the CspA binding sites for C7 and C9 overlap (64). More recently, work in a neurotropic genospecies of Lyme disease spirochetes called B. bavariensis identified the surface proteins BGA66 and BGA71 that directly bind to C7, C8, and C9 and prevent MAC formation (65). Like CspA, both of these proteins are members of the Pfam54 family, but unlike CspA they each fail to interact with human FH. Surprisingly, the crystal structure of BGA71 revealed a novel homodimer structure mediated by a disulfide bridge between BGA71 monomers (Figure 2) (40). As the dimerization of CspA is critical for FH-binding, the distinction in the oligomeric structures between CspA and BGA71 provides a plausible explanation for their difference in FH binding. This structure also provides a potential basis for the conserved nature of the MAC inhibitory activities between CspA and BGA71, due in part to a putative common C7-binding site (40).
By directly targeting C5b-9/MAC, and thus blocking the complement cascade at distal steps, CspA, BGA66, and BGA71 all act in a pathway-independent manner. B. burgdorferi also express at least two pathway-specific inhibitors that block the far upstream complement initiation steps. Caine et. al showed that OspC binds directly to C4b and prevents the formation CP/LP proconvertases (i.e. C4bC2) by preventing C2 binding (66). Interestingly, OspC selectively inhibits CP/LP activation using a mechanism similar to extracellular adherence protein (EAP) from Staphylococcus aureus and complement interfering protein (CIP) from group B Streptococcus (67, 68). Lyme disease spirochetes produce a second, albeit exclusive, CP inhibitor in the form of the lipoprotein BBK32. SPR assays showed that the C-terminal region of BBK32 (i.e. BBK32-C) bound with high-affinity to the first component of complement, C1, and inhibited its activation (69). Interestingly, BBK32-C is highly specific for the C1r subcomponent of the C1 complex (69). The three-dimensional structure of BBK32-C was recently solved by x-ray crystallography (Figure 2) and in the same study the BBK32 binding site on C1r was mapped to the C1r serine protease domain (70). When BBK32 was produced on the surface of a serum-sensitive strain of B. burgdorferi these spirochetes became significantly more serum resistant (69). However, the BBK32 homolog, termed BGD19, from a serum sensitive B. garinii strain, was significantly impaired in its ability to protect serum-sensitive B. burgdorferi from complement-mediated lysis (70).
The role of complement evasion proteins in pathogenesis
In order to link the complement resistance function of a borrelial protein observed in vitro to a pathogenic outcome, one would need to establish a role of said protein to survival in animal models of infection. One complication of several candidates discussed here are their redundancy within the borrelial genome (highlighted below), which limits the ability to link a complement resistant phenotype to a single genetic locus. For example, the presence of three distinct classes of FH-binding proteins (i.e. CRASPs) further complicates the determination of how an individual protein contributes to borrelial pathogenesis. One way to address this conundrum is to focus on temporal conditions where a single CRASP protein is expressed. Recently, Hart et al. demonstrated that B. burgdorferi cspA mutants could infect Ixodes ticks but were not transmitted during a murine blood meal, suggesting that CspA is needed at this time to protect B. burgdorferi from complement-dependent killing (39). This is consistent with CspA being made selectively within flat or feeding Ixodes nymphs relative to CspZ and the Erp proteins (71). The importance of the temporal nature of CRASP production was also seen when similar B. burgdorferi cspA mutants were tested for their infectivity potential following needle inoculation. Here, no apparent phenotype was observed which is consistent with concurrent production of CspZ, ErpP, and ErpA (71).
Evaluation of B. burgdorferi cspZ mutants by needle inoculation showed that CspZ was not required for murine infection, perhaps due to the compensatory function afforded by the presence of ErpP and ErpA (61, 71). However, incubation of borrelial cells in human blood stimulated production of CspZ and resulted in an increased differential in bacteremia and disseminated infection relative to the similarly treated B. burgdorferi cspZ mutant, suggesting that increased CspZ production bolsters the pathogenicity of B. burgdorferi (72). As such, the current temporal/spatial data suggests that CspA is needed for transmission from infected fed ticks to mammalian hosts while CspZ contributes to vertebrate infection, including dissemination. In contrast, the role(s) of ErpA, ErpP, or ErpC in experimental Lyme borreliosis remain unclear.
Of the Borreliella proteins that directly block complement components (other than CspA/MAC), only OspC and BBK32 have been evaluated for their role in borrelial pathogenesis via experimental infection. B. burgdorferi ospC mutants are well documented in their severe impairment to experimentally infect mice, including infectivity defects for individual site directed mutations in ospC (73-75). A B. burgdorferi ospC mutant showed reduced survival in blood at very early time points post-infection (i.e. 30 minutes), which is consistent with a role for OspC in complement evasion (66). For B. burgdorferi bbk32 mutants, the phenotype observed is complicated by the multifunctionality of BBK32. In this regard, B. burgdorferi lacking bbk32 are still infectious but are significantly attenuated (76, 77). Since BBK32 binds fibronectin and glycosoaminoglycans (GAG), the muted phenotype of the B. burgdorferi bbk32 mutant was initially thought to be associated with compensatory adherence since other borrelial proteins share this activity (78-81). However, the ability of BBK32 to inhibit the CP by binding to C1r is likely to contribute to the phenotype observed and implies that, similar to the redundant fibronectin and GAG binding observed for other borrelial proteins, additional B. burgdorferi proteins function to inhibit the CP in the absence of BBK32.
The redundant and multifunctional nature of Lyme disease spirochete complement evasion proteins
Two concepts consistently arise in the study of complement evasion mechanisms employed by Lyme disease spirochetes – specifically, redundancy and multifunctionality. Redundancy manifests in at least three ways, i) a form of functional redundancy whereby a paralogous gene product functions to target the same complement ligand (e.g. ErpA/ErpP/ErpC), ii) another form of functional redundancy where distinct gene products produce proteins that target the same intervention point or host complement pathway (e.g. CRASPs/FH or OspC/BBK32/CP), and iii) a form of genetic redundancy whereby a gene is encoded more than once in the Lyme disease spirochete genome (e.g. erpA (17, 82)). Multifunctionality is related to the observation that many proteins involved in borrelial complement evasion have more than one function within the host. As it relates to complement evasion, there are two types of multifunctionality: i) those proteins that interact with more than one complement ligand resulting in independent layers of complement inhibition (e.g. CspA/FH and CspA/MAC) and ii) those proteins that also interact with non-complement ligands. The latter is typified by BBK32 which harbors non-overlapping binding sites for glycosaminoglycans, fibronectin, and C1r (69, 79, 80, 83). Moreover, these two types of multifunctionality can be bridged. This is best appreciated by the reported interactions of several proteins discussed in this review (i.e. CspA, CspZ, ErpATVC, and OspC) with host plasminogen, which independently functions in the targeted degradation of host complement proteins (84-87).
Concluding Remarks and Future Perspectives
Lyme disease spirochetes produce a small arsenal of outer surface lipoproteins that specifically target and inactivate host complement (Table 1). Several proteins involved in borrelial complement evasion such as BBK32, OspC, BGA66, and BGA71 have only recently been described. CspA – which has been known to protect Lyme disease spirochetes from complement attack for nearly two decades – has only recently been revealed to harbor multiple mechanisms of complement inhibitory activity. Thus, it seems likely that the list of Borreliella proteins that contribute to host complement evasion will continue to grow. However, reconciling the paradox of an apparently robust and multilayered complement evasion system with the results from numerous experimental Lyme borreliosis studies, especially in complement deficient animals (Box 1), remains a key challenge for the future. The observation that borrelial complement evasion involves both multifunctional and redundant architecture presents several obstacles to addressing the role of individual complement evasion proteins to the pathogenesis of Lyme disease. As highlighted by studies reviewed here, progress in this area will require a mechanistic and multidisciplinary approach. In our view this effort is warranted as further research in this area stands to greatly improve our understanding of how important human pathogens like Lyme disease spirochetes survive and persist in immunocompetent hosts.
Box 1. Role of host complement in borrelial infectivity.
The role of complement in protecting hosts from infections with Lyme disease spirochetes has been investigated predominantly using mice that are genetically deficient in one or more complement components. In this regard, complement component C3 has been studied most extensively. Woodman et. al found that when mice deficient in C3 are infected with B. burgdorferi a significant difference in bacterial loads could only be detected in the ears of mice two weeks post infection, whereas no significant differences were found in other tissues or timepoints (88). Similarly, van Burgel et al. showed that C3 deficient mice infected with B. burgdorferi, B. afzelii, or B. bavariensis showed no significant differences compared to wild-type mice with the exception of increased B. burgdorferi load in joints (89). However, Lawrenz, et al. showed that spirochetal loads were higher at several time points and in multiple tissues in C3 deficient mice and that this increased bacterial burden correlated with earlier development of arthritis (90). In line with this study, an in vivo role for C3 in control of B. burgdorferi was demonstrated in a hamster model of infection where treatment with the decomplementation reagent, cobra venom factor, was used (91).
While C3 activation is central to the complement cascade, the role of the upstream initiation of complement and distal reactions of complement have also been evaluated in murine infections. Infection of B. burgdorferi in C5 deficient mice found that C5 is not required to control murine borreliosis (92). Similarly, when key AP-associated components FB and FH have been knocked out in mice, no significant differences in B. burgdorferi bacterial loads were detected (88). In contrast, an early protective role was shown for MBL in mice in both needle-and tick infection models (93). However, no differences in dissemination were observed in MBL-deficient mice relative to its parent strain (93). The role of CP initiation in response to B. burgdorferi infection was recently investigated using C1q deficient mice (94). Significantly increased bacterial loads were measured at several time points post infection and in multiple tissue types. In contrast to the studies conducted with MBL deficient mice, C1q deficient mice exhibited altered cytokine profiles relative to wild-type controls (94). Collectively, studies involving complement deficient mice indicate a potential role in experimental Lyme borreliosis for the upstream initiation steps of complement, a modest role for C3, and a more limited role for the AP and C5 activation in controlling infection by Lyme disease spirochetes.
Outstanding Questions.
Given the apparent functional redundancy of the Lyme disease complement evasion system, are there other outer surface proteins that have unrecognized roles in protecting Lyme disease spirochetes from complement attack?
The involvement of complement in human autoimmune and inflammatory diseases is well established. Can detailed mechanistic knowledge of Lyme disease complement evasion strategies lead to the development of new complement directed therapies?
How do the collective activities of Lyme disease spirochete complement evasion proteins contribute to vertebrate-specific host association?
How do the various complement evasion proteins produced by Lyme disease spirochetes impact the adaptive immune response during borreliosis?”
What is the role for Lyme disease spirochete complement evasion proteins in persistence?
What are potential reasons for the seemingly paradoxical retention of a large complement evasion arsenal within the Lyme disease spirochete genome, and data from complement deficient murine infection models, which suggest an overall limited role for complement in controlling experimental Lyme borreliosis?
Given the reported differences in binding specificities and the differential gene expression of CspA, CspZ, and ErpA/ErpP/ErpC, what might be the physiological role of FHL and FHR proteins in Lyme borreliosis?
How well conserved are complement evasion mechanisms across relapsing fever Borrelia?
Highlights.
Borreliella species encode a wide variety of surface proteins that neutralize complement-dependent killing pathways.
The complement evasion system of Lyme spirochetes is multipronged and functionally overlapping.
Recent work in the field of structural biology has revealed new insight into the molecular basis for the diverse activities of several outer surface proteins involved in Lyme disease-specific complement evasion.
Acknowledgements
Complement evasion-based research in the authors laboratories is supported by Public Health Service grants AI-133367 and AI-146930 from the National Institute of Allergy and Infectious Diseases. We thank Alexandra D. Powell for help with Figure 1. Due to space restrictions, the authors acknowledge that all contributions in this area of borrelial research could not be included in this review.
Glossary
- Alternative pathway (AP)
One of the three conventional activation pathways of complement. The AP is under continuous activation by a process called tick-over whereby a labile thioester bond in fluid phase C3 hydrolyzes to form C3(H2O) that complexes with factors B and D to generate active AP C3 convertases.
- C1
The first component of complement, C1, is a multiprotein complex composed of C1q and a heterotrimer of the serine proteases C1r and C1s (i.e. C1qr2s2). C1 activates the CP by the pattern recognition activity of C1q and the initiating protease activities of C1r and C1s.
- Classical pathway (CP)
One of the three conventional activation pathways of complement. The CP is activated by C1 following binding of C1q to IgM or IgG immune complexes, or nonantibody activating ligands.
- Convertase
Multiprotein complexes of the complement system consisting of a surface-bound scaffolding component bound to a protease. Convertases act to convert inert C3 or C5 to activated fragments. C3 convertases take the form C3bBb and C4b2b. On surfaces with high local C3b densities, C3 convertases bind an additional C3b molecule and switch substrate specificity from C3 to C5.
- Complement regulator-acquiring surface proteins (CRASPs)
Term collectively used to describe the distinct FH-binding proteins produced by Lyme disease spirochetes. The group is composed of CRASP-1 (CspA), CRASP-2 (CspZ), CRASP-3 (ErpP), CRASP-4 (ErpC), and CRASP-5 (ErpA).
- Factor H (FH)
FH is the major negative regulator of the AP. FH binds to C3b and competes with factor B for proconvertase formation and stimulates the nonreversible release of the protease Bb fragment (i.e. decay accelerating activity). FH also serves as a cofactor for the factor-I mediated degradation of C3b.
- Lectin pathway (LP)
One of the three conventional pathways of complement. The LP is initiated by the carbohydrate-binding pattern recognition molecules MBL, ficolins, collectin-10, and collectin-11 which circulate in complex with the LP initiating proteases known as the MBLassociated proteases (MASPs).
- Lipoprotein
A class of proteins that are covalently anchored to bacterial membranes by N-terminal lipid modifications.
- Membrane attack complex (MAC)
A lytic pore structure formed by a multiprotein complex composed of C5b, C6, C7, C8, and multiple copies of C9. MAC, also known as C5b-9, or the terminal pathway complete complex, is formed following C5 cleavage by convertases. MAC formation can result in direct lysis of cells and is the lytic component of serum.
- Pfam54
The largest paralogous gene family present in the Lyme disease spirochete genome containing up to eleven members. Several Pfam54 genes are involved in complement evasion including CspA, BGA66 and BGA71.
- Regulators of complement activity (RCAs)
RCAs are proteins that regulate complement activation in order to protect healthy host tissues from inappropriate complement targeting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuehn BM 2013. CDC Estimates 300 000 US Cases of Lyme Disease Annually. Jama 310: 1110. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg R 2018. Vital Signs: Trends in Reported Vectorborne Disease Cases — United States and Territories, 2004–2016. MMWR Morb Mortal Wkly Rep 67: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, and Mead PS. 2014. Lyme Disease Testing by Large Commercial Laboratories in the United States. Clinical Infectious Diseases 59: 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radolf JD, Caimano MJ, Stevenson B, and Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol 10: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adeolu M and Gupta RS. 2014. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: the emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Van Leeuwenhoek 105: 1049–1072. [DOI] [PubMed] [Google Scholar]
- 6.Steere AC 2001. Lyme disease. N. Engl. J. Med 345: 115–125. [DOI] [PubMed] [Google Scholar]
- 7.Steere AC, Coburn J, and Glickstein L. 2004. The emergence of Lyme disease. J. Clin. Invest 113: 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro ED 2014. Lyme disease. N. Engl. J. Med 371: 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wormser GP, McKenna D, Carlin J, Nadelman RB, Cavaliere LF, Holmgren D, Byrne DW, and Nowakowski J. 2005. Brief communication: hematogenous dissemination in early Lyme disease. Ann. Intern. Med 142: 751–755. [DOI] [PubMed] [Google Scholar]
- 10.Merle NS, Church SE, Fremeaux-Bacchi V, and Roumenina LT. 2015. Complement System Part I - Molecular Mechanisms of Activation and Regulation. Front Immunol 6: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, and Roumenina LT. 2015. Complement System Part II: Role in Immunity. Front Immunol 6: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt CQ, Lambris JD, and Ricklin D. 2016. Protection of host cells by complement regulators. Immunol. Rev 274: 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breitner-Ruddock S, Wurzner R, Schulze J, and Brade V. 1997. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med. Microbiol. Immunol 185: 253–260. [DOI] [PubMed] [Google Scholar]
- 14.van Dam AP, Oei A, Jaspars R, Fijen C, Wilske B, Spanjaard L, and Dankert J. 1997. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect. Immun 65: 1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraiczy P 2016. Hide and Seek: How Lyme Disease Spirochetes Overcome Complement Attack. Frontiers in Immunology 7: 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bykowski T, Woodman ME, Cooley AE, Brissette CA, Wallich R, Brade V, Kraiczy P, and Stevenson B. 2008. Borrelia burgdorferi complement regulator-acquiring surface proteins (BbCRASPs): Expression patterns during the mammal-tick infection cycle. Int. J. Med. Microbiol 298 Suppl 1: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraiczy P and Stevenson B. 2013. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: Structure, function and regulation of gene expression. Ticks Tick Borne Dis 4: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Taeye SW, Kreuk L, van Dam AP, Hovius JW, and Schuijt TJ. 2013. Complement evasion by Borrelia burgdorferi: it takes three to tango. Trends Parasitol. 29: 119–128. [DOI] [PubMed] [Google Scholar]
- 19.Stone BL and Brissette CA. 2017. Host Immune Evasion by Lyme and Relapsing Fever Borreliae: Findings to Lead Future Studies for Borrelia miyamotoi. Front Immunol 8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caine JA and Coburn J. 2016. Multifunctional and Redundant Roles of Borrelia burgdorferi Outer Surface Proteins in Tissue Adhesion, Colonization, and Complement Evasion. Front Immunol 7: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraiczy P 2016. Travelling between Two Worlds: Complement as a Gatekeeper for an Expanded Host Range of Lyme Disease Spirochetes. Vet Sci 3:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locke JW 2019. Complement Evasion in Borrelia spirochetes: Mechanisms and Opportunities for Intervention. Antibiotics (Basel) 8:e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y, Frye AM, Nowak TA, and Kraiczy P. 2020. New Insights Into CRASPMediated Complement Evasion in the Lyme Disease Enzootic Cycle. Front. Cell. Infect. Microbiol 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietikäinen J, Meri T, Blom AM, and Meri S. 2010. Binding of the complement inhibitor C4b-binding protein to Lyme disease Borreliae. Mol. Immunol 47: 1299–1305. [DOI] [PubMed] [Google Scholar]
- 25.Pausa M, Pellis V, Cinco M, Giulianini PG, Presani G, Perticarari S, Murgia R, and Tedesco F. 2003. Serum-resistant strains of Borrelia burgdorferi evade complement-mediated killing by expressing a CD59-like complement inhibitory molecule. J. Immunol 170: 3214–3222. [DOI] [PubMed] [Google Scholar]
- 26.Weiler JM, Daha MR, Austen KF, and Fearon DT. 1976. Control of the amplification convertase of complement by the plasma protein beta1H. Proc. Natl. Acad. Sci. U. S. A 73: 3268–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pangburn MK, Schreiber RD, and Muller-Eberhard HJ. 1977. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J. Exp. Med 146: 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makou E, Herbert AP, and Barlow PN. 2013. Functional anatomy of complement factor H. Biochemistry 52: 3949–3962. [DOI] [PubMed] [Google Scholar]
- 29.Gordon DL, Kaufman RM, Blackmore TK, Kwong J, and Lublin DM. 1995. Identification of complement regulatory domains in human factor H. J. Immunol 155: 348–356. [PubMed] [Google Scholar]
- 30.Kraiczy P, Skerka C, Brade V, and Zipfel PF. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun 69: 7800–7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraiczy P, Hellwage J, Skerka C, Becker H, Kirschfink M, Simon MM, Brade V, Zipfel PF, and Wallich R. 2004. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem 279: 2421–2429. [DOI] [PubMed] [Google Scholar]
- 32.Brooks CS, Vuppala SR, Jett AM, Alitalo A, Meri S, and Akins DR. 2005. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J. Immunol 175: 3299–3308. [DOI] [PubMed] [Google Scholar]
- 33.Kenedy MR, Vuppala SR, Siegel C, Kraiczy P, and Akins DR. 2009. CspA-mediated binding of human factor H inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect. Immun 77: 2773–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallich R, Pattathu J, Kitiratschky V, Brenner C, Zipfel PF, Brade V, Simon MM, and Kraiczy P. 2005. Identification and functional characterization of complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes Borrelia afzelii and Borrelia garinii. Infect. Immun 73: 2351–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordes FS, Roversi P, Kraiczy P, Simon MM, Brade V, Jahraus O, Wallis R, Skerka C, Zipfel PF, Wallich R, and Lea SM. 2005. A novel fold for the factor H-binding protein BbCRASP-1 of Borrelia burgdorferi. Nat. Struct. Mol. Biol 12: 276–277. [DOI] [PubMed] [Google Scholar]
- 36.Caesar JJE, Wallich R, Kraiczy P, Zipfel PF, and Lea SM. 2013. Further structural insights into the binding of complement factor H by complement regulator-acquiring surface protein 1 (CspA) of Borrelia burgdorferi. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun 69: 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraiczy P, Hanssen-Hubner C, Kitiratschky V, Brenner C, Besier S, Brade V, Simon MM, Skerka C, Roversi P, Lea SM, Stevenson B, Wallich R, and Zipfel PF. 2009. Mutational analyses of the BbCRASP-1 protein of Borrelia burgdorferi identify residues relevant for the architecture and binding of host complement regulators FHL-1 and factor H. Int. J. Med. Microbiol 299: 255–268. [DOI] [PubMed] [Google Scholar]
- 38.Wywial E, Haven J, Casjens SR, Hernandez YA, Singh S, Mongodin EF, Fraser-Liggett CM, Luft BJ, Schutzer SE, and Qiu W. 2009. Fast, adaptive evolution at a bacterial host-resistance locus: the PFam54 gene array in Borrelia burgdorferi. Gene 445: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart T, Nguyen NTT, Nowak NA, Zhang F, Linhardt RJ, Diuk-Wasser M, Ram S, Kraiczy P, and Lin Y. 2018. Polymorphic factor H-binding activity of CspA protects Lyme borreliae from the host complement in feeding ticks to facilitate tick-to-host transmission. PLoS Pathog. 14: e1007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brangulis K, Akopjana I, Petrovskis I, Kazaks A, and Tars K. 2019. Crystal structure of Borrelia burgdorferi outer surface protein BBA69 in comparison to the paralogous protein CspA. Ticks Tick Borne Dis 10: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 41.Hartmann K, Corvey C, Skerka C, Kirschfink M, Karas M, Brade V, Miller JC, Stevenson B, Wallich R, Zipfel PF, and Kraiczy P. 2006. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol. Microbiol 61: 1220–1236. [DOI] [PubMed] [Google Scholar]
- 42.Siegel C, Schreiber J, Haupt K, Skerka C, Brade V, Simon MM, Stevenson B, Wallich R, Zipfel PF, and Kraiczy P. 2008. Deciphering the ligand-binding sites in the Borrelia burgdorferi complement regulator-acquiring surface protein 2 required for interactions with the human immune regulators factor H and factor H-like protein 1. J. Biol. Chem 283: 34855–34863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brangulis K, Petrovskis I, Kazaks A, Bogans J, Otikovs M, Jaudzems K, Ranka R, and Tars K. 2014. Structural characterization of CspZ, a complement regulator factor H and FHL-1 binding protein from Borrelia burgdorferi. Febs J. 281: 2613–2622. [DOI] [PubMed] [Google Scholar]
- 44.Haupt K, Kraiczy P, Wallich R, Brade V, Skerka C, and Zipfel PF. 2007. Binding of human factor H-related protein 1 to serum-resistant Borrelia burgdorferi is mediated by borrelial complement regulator-acquiring surface proteins. J. Infect. Dis 196: 124–133. [DOI] [PubMed] [Google Scholar]
- 45.Brangulis K, Petrovskis I, Kazaks A, Akopjana I, and Tars K. 2015. Crystal structures of the Erp protein family members ErpP and ErpC from Borrelia burgdorferi reveal the reason for different affinities for complement regulator factor H. Biochim. Biophys. Acta 1854: 349–355. [DOI] [PubMed] [Google Scholar]
- 46.Bhattacharjee A, Oeemig JS, Kolodziejczyk R, Meri T, Kajander T, Lehtinen MJ, Iwa’i H, Jokiranta TS, and Goldman A. 2013. Structural basis for complement evasion by Lyme disease pathogen Borrelia burgdorferi. J. Biol. Chem 288: 18685–18695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolodziejczyk R, Mikula KM, Kotila T, Postis VLG, Jokiranta TS, Goldman A, and Meri T. 2017. Crystal structure of a tripartite complex between C3dg, C-terminal domains of factor H and OspE of Borrelia burgdorferi. PLoS ONE 12: e0188127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammerschmidt C, Hallstrom T, Skerka C, Wallich R, Stevenson B, Zipfel PF, and Kraiczy P. 2012. Contribution of the infection-associated complement regulator-acquiring surface protein 4 (ErpC) to complement resistance of Borrelia burgdorferi. Clin. Dev. Immunol 2012: 349657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siegel C, Hallstrom T, Skerka C, Eberhardt H, Uzonyi B, Beckhaus T, Karas M, Wallich R, Stevenson B, Zipfel PF, and Kraiczy P. 2010. Complement factor H-related proteins CFHR2 and CFHR5 represent novel ligands for the infection-associated CRASP proteins of Borrelia burgdorferi. PLoS ONE 5: e13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kenedy MR and Akins DR. 2011. The OspE-related proteins inhibit complement deposition and enhance serum resistance of Borrelia burgdorferi, the Lyme disease spirochete. Infect. Immun 79: 1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kühn S, Skerka C, and Zipfel PF. 1995. Mapping of the complement regulatory domains in the human factor H-like protein 1 and in factor H1. J. Immunol 155: 5663–5670. [PubMed] [Google Scholar]
- 52.Pangburn MK 2002. Cutting edge: localization of the host recognition functions of complement factor H at the carboxyl-terminal: implications for hemolytic uremic syndrome. J. Immunol 169: 4702–4706. [DOI] [PubMed] [Google Scholar]
- 53.Meri T, Amdahl H, Lehtinen MJ, Hyvärinen S, McDowell JV, Bhattacharjee A, Meri S, Marconi R, Goldman A, and Jokiranta TS. 2013. Microbes bind complement inhibitor factor H via a common site. PLoS Pathog. 9: e1003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kraiczy P, Hellwage J, Skerka C, Kirschfink M, Brade V, Zipfel PF, and Wallich R. 2003. Immune evasion of Borrelia burgdorferi: mapping of a complement-inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur. J. Immunol 33: 697–707. [DOI] [PubMed] [Google Scholar]
- 55.Schneider MC, Prosser BE, Caesar JJE, Kugelberg E, Li S, Zhang Q, Quoraishi S, Lovett JE, Deane JE, Sim RB, Roversi P, Johnson S, Tang CM, and Lea SM. 2009. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature 458: 890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blom AM, Hallstrom T, and Riesbeck K. 2009. Complement evasion strategies of pathogens-acquisition of inhibitors and beyond. Mol. Immunol 46: 2808–2817. [DOI] [PubMed] [Google Scholar]
- 57.Hovingh ES, van den Broek B, and Jongerius I. 2016. Hijacking Complement Regulatory Proteins for Bacterial Immune Evasion. Front Microbiol 7: 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Lackum K, Miller JC, Bykowski T, Riley SP, Woodman ME, Brade V, Kraiczy P, Stevenson B, and Wallich R. 2005. Borrelia burgdorferi regulates expression of complement regulator-acquiring surface protein 1 during the mammal-tick infection cycle. Infect. Immun 73: 7398–7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDowell JV, Hovis KM, Zhang H, Tran E, Lankford J, and Marconi RT. 2006. Evidence that the BBA68 protein (BbCRASP-1) of the Lyme disease spirochetes does not contribute to factor H-mediated immune evasion in humans and other animals. Infect. Immun 74: 3030–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lederer S, Brenner C, Stehle T, Gem L, Wallich R, and Simon MM. 2005. Quantitative analysis of Borrelia burgdorferi gene expression in naturally (tick) infected mouse strains. Med. Microbiol. Immunol 194: 81–90. [DOI] [PubMed] [Google Scholar]
- 61.Coleman AS, Yang X, Kumar M, Zhang X, Promnares K, Shroder D, Kenedy MR, Anderson JF, Akins DR, and Pal U. 2008. Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS ONE 3: 3010e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cserhalmi M, Papp A, Brandus B, Uzonyi B, and Józsi M. 2019. Regulation of regulators: Role of the complement factor H-related proteins. Semin. Immunol 45: 101341. [DOI] [PubMed] [Google Scholar]
- 63.Irmscher S, Brix SR, Zipfel SLH, Halder LD, Mutluturk S, Wulf S, Girdauskas E, Reichenspurner H, Stahl RAK, Jungnickel B, Wiech T, Zipfel PF, and Skerka C. 2019. Serum FHR1 binding to necrotic-type cells activates monocytic inflammasome and marks necrotic sites in vasculopathies. Nat Commun 10: 2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hallström T, Siegel C, Morgelin M, Kraiczy P, Skerka C, and Zipfel PF. 2013. CspA from Borrelia burgdorferi inhibits the terminal complement pathway. mBio 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hammerschmidt C, Klevenhaus Y, Koenigs A, Hallstrom T, Fingerle V, Skerka C, Pos KM, Zipfel PF, Wallich R, and Kraiczy P. 2016. BGA66 and BGA71 facilitate complement resistance of Borrelia bavariensis by inhibiting assembly of the membrane attack complex. Mol. Microbiol 99: 407–424. [DOI] [PubMed] [Google Scholar]
- 66.Caine JA, Lin Y, Kessler JR, Sato H, Leong JM, and Cobum J. 2017. Borrelia burgdorferi outer surface protein C (OspC) binds complement component C4b and confers bloodstream survival. Cell. Microbiol. 19:e12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woehl JL, Stapels DA, Garcia BL, Ramyar KX, Keightley A, Ruyken M, Syriga M, Sfyroera G, Weber AB, Zolkiewski M, Ricklin D, Lambris JD, Rooijakkers SH, and Geisbrecht BV. 2014. The Extracellular Adherence Protein from Staphylococcus aureus Inhibits the Classical and Lectin Pathways of Complement by Blocking Formation of the C3 Proconvertase. Journal of Immunology 193: 6161–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pietrocola G, Rindi S, Rosini R, Buccato S, Speziale P, and Margarit I. 2016. The Group B Streptococcus-Secreted Protein CIP Interacts with C4, Preventing C3b Deposition via the Lectin and Classical Complement Pathways. J. Immunol 196: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia BL, Zhi H, Wager B, Hook M, and Skare JT. 2016. Borrelia burgdorferi BBK32 Inhibits the Classical Pathway by Blocking Activation of the C1 Complement Complex. PLoS Pathogens 12: e1005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie J, Zhi H, Garrigues RJ, Keightley A, Garcia BL, and Skare JT. 2019. Structural determination of the complement inhibitory domain of Borrelia burgdorferi BBK32 provides insight into classical pathway complement evasion by Lyme disease spirochetes. PLoS Pathog 15: el007659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bykowski T, Woodman ME, Cooley AE, Brissette CA, Brade V, Wallich R, Kraiczy P, and Stevenson B. 2007. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete's mammal-tick infection cycle. Infect. Immun 75: 4227–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marcinkiewicz AL, Dupuis AP, Zamba-Campero M, Nowak N, Kraiczy P, Ram S, Kramer LD, and Lin Y. 2019. Blood treatment of Lyme borreliae demonstrates the mechanism of CspZ-mediated complement evasion to promote systemic infection in vertebrate hosts. Cellular Microbiology 21: e12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, and Rosa PA. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U. S. A 101: 3142–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, Flavell RA, Norgard MV, and Fikrig E. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Invest 113: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Earnhart CG, Leblanc DV, Alix KE, Desrosiers DC, Radolf JD, and Marconi RT. 2010. Identification of residues within ligand-binding domain 1 (LBD1) of the Borrelia burgdorferi OspC protein required for function in the mammalian environment. Mol. Microbiol 76: 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, Höök M, and Skare JT. 2006. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol 59: 1591–1601. [DOI] [PubMed] [Google Scholar]
- 77.Hyde JA, Weening EH, Chang M, Trzeciakowski JP, Hook M, Cirillo JD, and Skare JT. 2011. Bioluminescent imaging of Borrelia burgdorferi in vivo demonstrates that the fibronectin-binding protein BBK32 is required for optimal infectivity. Mol. Microbiol 82: 99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gaultney RA, Gonzalez T, Floden AM, and Brissette CA. 2013. BB0347, from the Lyme disease spirochete Borrelia burgdorferi, is surface exposed and interacts with the CS1 heparin-binding domain of human fibronectin. PLoS ONE 8: e75643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fischer JR, LeBlanc KT, and Leong JM. 2006. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect. Immun 74: 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin Y, Chen Q, Ritchie JA, Dufour NP, Fischer JR, Coburn J, and Leong JM. 2015. Glycosaminoglycan binding by Borrelia burgdorferi adhesin BBK32 specifically and uniquely promotes joint colonization. Cell. Microbiol 17: 860–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brissette CA, Bykowski T, Cooley AE, Bowman A, and Stevenson B. 2009. Borrelia burgdorferi RevA antigen binds host fibronectin. Infect. Immun 77: 2802–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stevenson B and Miller JC. 2003. Intra- and interbacterial genetic exchange of Lyme disease spirochete erp genes generates sequence identity amidst diversity. J. Mol. Evol 57: 309–324. [DOI] [PubMed] [Google Scholar]
- 83.Probert WS and Johnson BJ. 1998. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol. Microbiol 30: 1003–1015. [DOI] [PubMed] [Google Scholar]
- 84.Hammerschmidt C, Koenigs A, Siegel C, Hallström T, Skerka C, Wallich R, Zipfel PF, and Kraiczy P. 2014. Versatile roles of CspA orthologs in complement inactivation of serum-resistant Lyme disease spirochetes. Infect. Immun 82: 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Önder Ö, Humphrey PT, McOmber B, Korobova F, Francella N, Greenbaum DC, and Brisson D. 2012. OspC is potent plasminogen receptor on surface of Borrelia burgdorferi. J. Biol. Chem 287: 16860–16868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hallstrom T, Haupt K, Kraiczy P, Hortschansky P, Wallich R, Skerka C, and Zipfel PF. 2010. Complement regulator-acquiring surface protein 1 of Borrelia burgdorferi binds to human bone morphogenic protein 2, several extracellular matrix proteins, and plasminogen. J. Infect. Dis 202: 490–498. [DOI] [PubMed] [Google Scholar]
- 87.Brissette CA, Haupt K, Barthel D, Cooley AE, Bowman A, Skerka C, Wallich R, Zipfel PF, Kraiczy P, and Stevenson B. 2009. Borrelia burgdorferi infection-associated surface proteins ErpP, ErpA, and ErpC bind human plasminogen. Infect. Immun. 77: 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woodman ME Cooley AE, Miller JC, Lazarus JJ, Tucker K, Bykowski T, Botto M, Hellwage J, Wooten RM, and Stevenson B. 2007. Borrelia burgdorferi Binding of Host Complement Regulator Factor H Is Not Required for Efficient Mammalian Infection. Infect. Immun 75: 3131–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Burgel ND, Balmus NCM, Fikrig E, and van Dam AP. 2011. Infectivity of Borrelia burgdorferi sensu lato is unaltered in C3-deficient mice. Ticks and Tick-Borne Diseases 2: 20–26. [DOI] [PubMed] [Google Scholar]
- 90.Lawrenz MB, Wooten RM, Zachary JF, Drouin SM, Weis JJ, Wetsel RA, and Norris SJ. 2003. Effect of Complement Component C3 Deficiency on Experimental Lyme Borreliosis in Mice. Infection and Immunity 71: 4432–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmitz JL, Lovrich SD, Callister SM, and Schell RF. 1991. Depletion of complement and effects on passive transfer of resistance to infection with Borrelia burgdorferi. Infect. Immun 59: 3815–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bockenstedt LK, Barthold S, Deponte K, Marcantonio N, and Kantor FS. 1993. Borrelia burgdorferi infection and immunity in mice deficient in the fifth component of complement. Infect. Immun 61: 2104–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coumou J, Wagemakers A, Narasimhan S, Schuijt TJ, Ersoz JI, Oei A, de Boer OJ, Roelofs Joris J. T. H., Fikrig E, and Hovius JW. 2019. The role of Mannose Binding Lectin in the immune response against Borrelia burgdorferi sensu lato. Sci Rep 9: 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhi H, Xie J, and Skare JT. 2018. The Classical Complement Pathway Is Required to Control Borrelia burgdorferi Levels During Experimental Infection. Frontiers in Immunology 9:959. [DOI] [PMC free article] [PubMed] [Google Scholar]