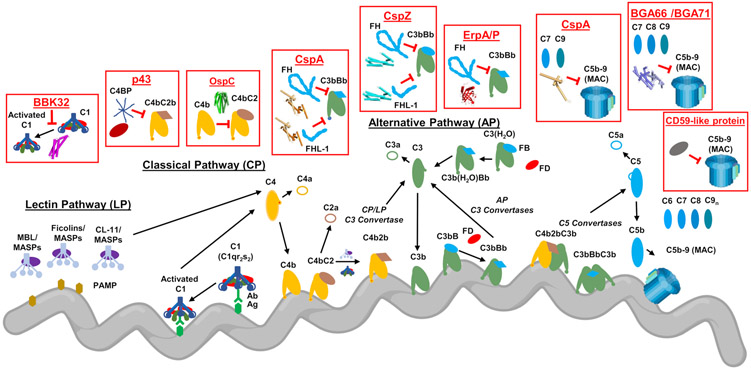
Figure 1. Complement Evasion by Lyme Disease Spirochetes.
To evade complement, Lyme disease spirochetes produce outer surface lipoproteins that bind directly to complement components. These proteins either inhibit complement activity directly or bind to host-derived regulators of complement activity (RCA) and thereby attenuate complement activation at the spirochete surface. Several of these inhibitors block the upstream initiation steps of the cascade including the CP-specific inhibitor BBK32, which binds to C1r within the C1 complex and traps C1 in a zymogen state. OspC binds C4b and interferes with the activation of both the CP and LP by preventing formation of the CP/LP C3 proconvertase (i.e. C4bC2). Lyme disease spirochetes also produce an outer surface protein of unknown identity termed p43 that downregulates the CP and LP by recruiting the primary RCA of these two pathways called C4b-binding protein (C4BP). CspA is among a group of three structurally unrelated proteins that bind the dominant negative regulator of the AP known as factor H (FH). CspA also binds to factor H-like protein 1 (FHL-1), a molecule of the FH family that retains complement regulatory activities. CspZ, a second FH/FHL-1-binding protein, also downregulates the formation of AP C3 and C5 convertases on the spirochete surface. A third type of FH-binding protein are the paralogs ErpA/ErpP/ErpC. Among these, ErpA and ErpP bind to FH (but not to FHL-1). Borreliella produce at least four proteins that block the formation of the MAC complex. CspA binds C7 and C9 in a FH-independent manner and blocks C9 polymerization. Two homologous proteins from B. bavariensis, BGA66 and BGA71 also block C9 polymerization by binding to C7, C8, and C9. Finally, an unidentified outer surface protein is produced by Lyme disease spirochetes that exhibits similar activity to the RCA known as CD59. PAMP: pathogen-associated molecular pattern; Ab: antibody; Ag: antigen; CL-11: collectin-11. This figure was created using BioRender.com.