Figure 2.
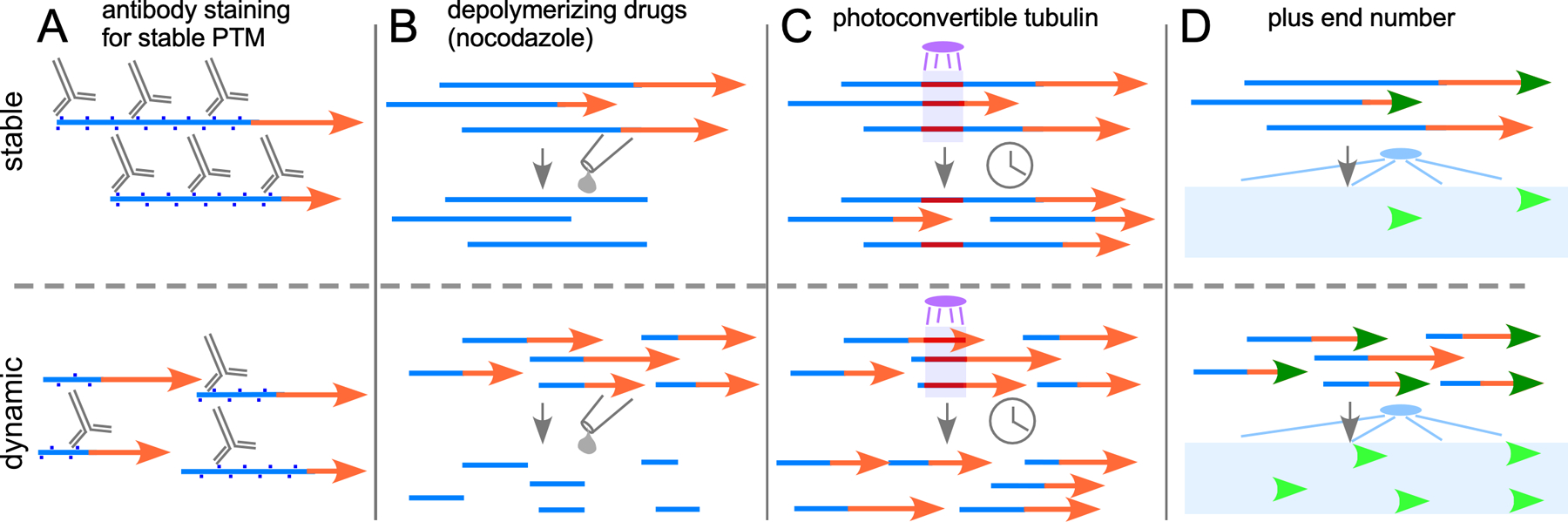
Assays to probe neuronal microtubule stability. (A) Antibodies that recognize PTMs associated with stable (blue) regions of microtubules can be used to get a relative idea of the amount of stable polymer in a region. Assuming the total amount of polymerized tubulin is similar, more antibody-binding is associated with microtubules with long stable regions (top) compared to shorter more dynamic microtubules (bottom). (B) Drugs that depolymerize microtubules initially cause loss of dynamic regions (orange) as they cycle through catastrophe and rescue. For long stable microtubules relatively little polymer is destabilized at early time points (top), while regions with short microtubules (bottom) show a greater loss. (C) Incorporation of photoconvertible, for example tdEOS, α-tubulin into microtubules allows conversion of a region of the microtubule to red over a green background using UV light. The red tubulin is trapped in the microtubule as long as it remains polymerized. Long microtubules may take hours to depolymerize back through stable regions and so the converted mark will remain (top), while complete depolymerization occurs on a much shorter timescale with more dynamic microtubules eliminating the red mark sooner (bottom). (D) Use of a labeled +TIP protein like EB1 allows visualization of each plus end that is growing, and each individual microtubule will cycle through periods of growth and shrinkage as it undergoes dynamic instability. With long stable microtubules relatively few growing plus ends will be seen at any one time (top), while shorter more dynamic microtubules will result in many more visible plus end comets (bottom).
