Figure 7.
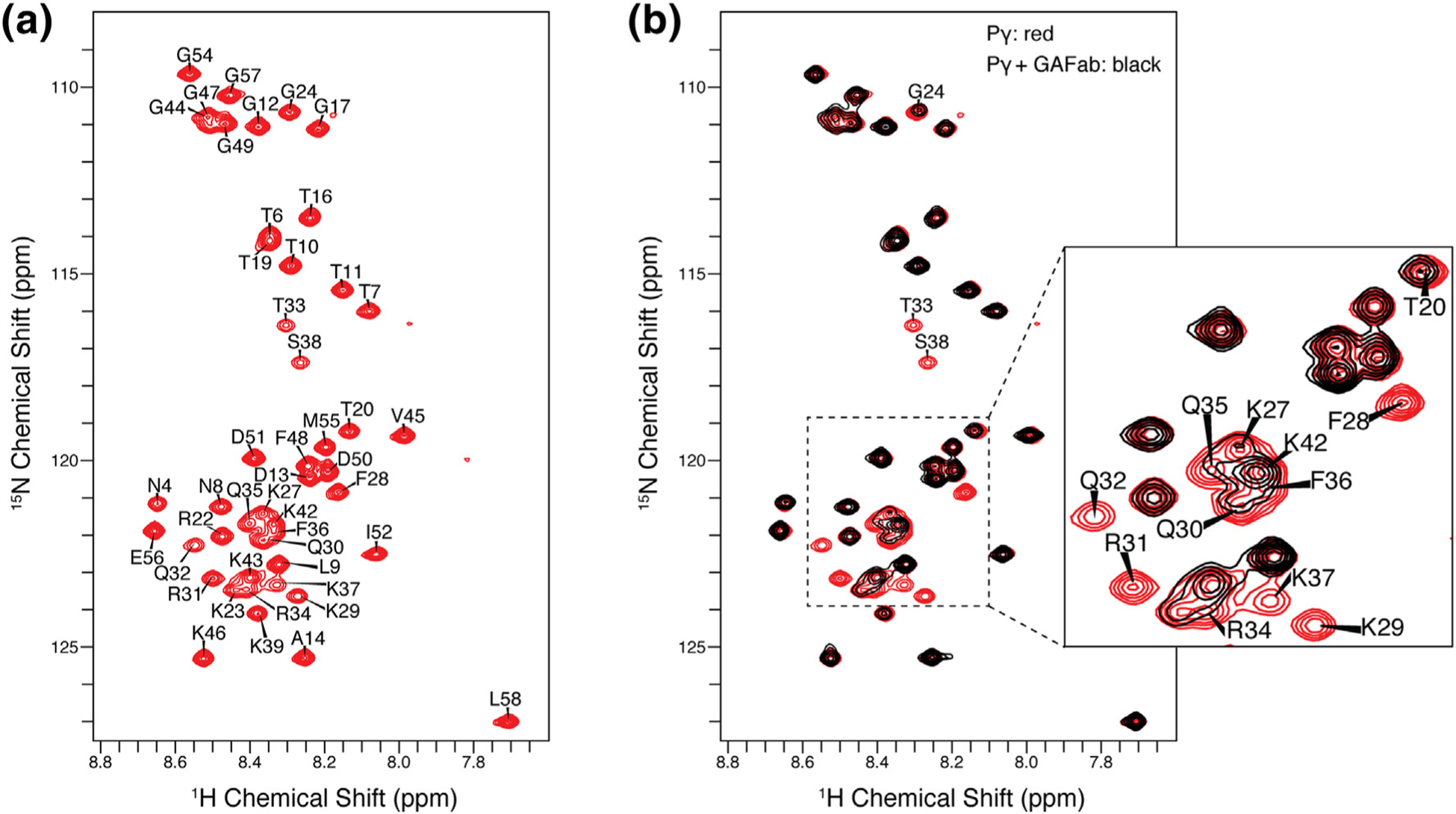
Pγ1–58 NMR resonance assignments and binding studies. (a) 2D 1H–15N HSQC NMR spectrum of Pγ in its unbound state. Narrow dispersion of 1H chemical shifts between 9.0 and 7.5 ppm is indicative of an intrinsically disordered protein. (b) The overlay of the 2D 1H–15N HSQC NMR spectra of the unbound Pγ (red spectrum) and Pγ bound to GAFab (black spectrum) illustrates that the addition of GAFab induced significant changes in the Pγ spectrum. The most significant line broadening was observed in the F28–S38 region of Pγ, indicative of the binding interface. Smaller signal attenuation was seen for neighboring residues and the C-terminal region.
