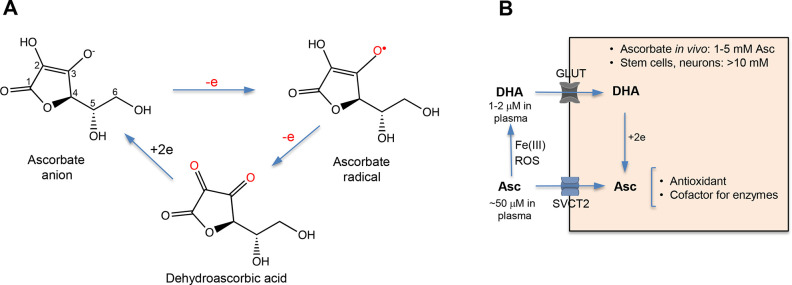
Figure 1.
Chemical forms and cellular metabolism of vitamin C. (A) Structures of the main forms of vitamin C. At physiological pH, reduced vitamin C predominantly exists as ascorbate anion due to its low pKa = 4.2. (B) Cellular uptake of reduced (ascorbate, Asc) and oxidized (dehydroascorbic acid, DHA) forms of vitamin C. Extracellular concentrations of dehydroascorbic acid and, consequently, the importance of its uptake are higher for cells that release large amounts of oxidants during the inflammatory responses (neutrophils, for example). Extracellular reduction of Fe(III) by ascorbate is another source of oxidized vitamin C which enters cells through GLUT glucose transporters. Fe(II) is taken up by cells via the divalent metal transporter DMT1.