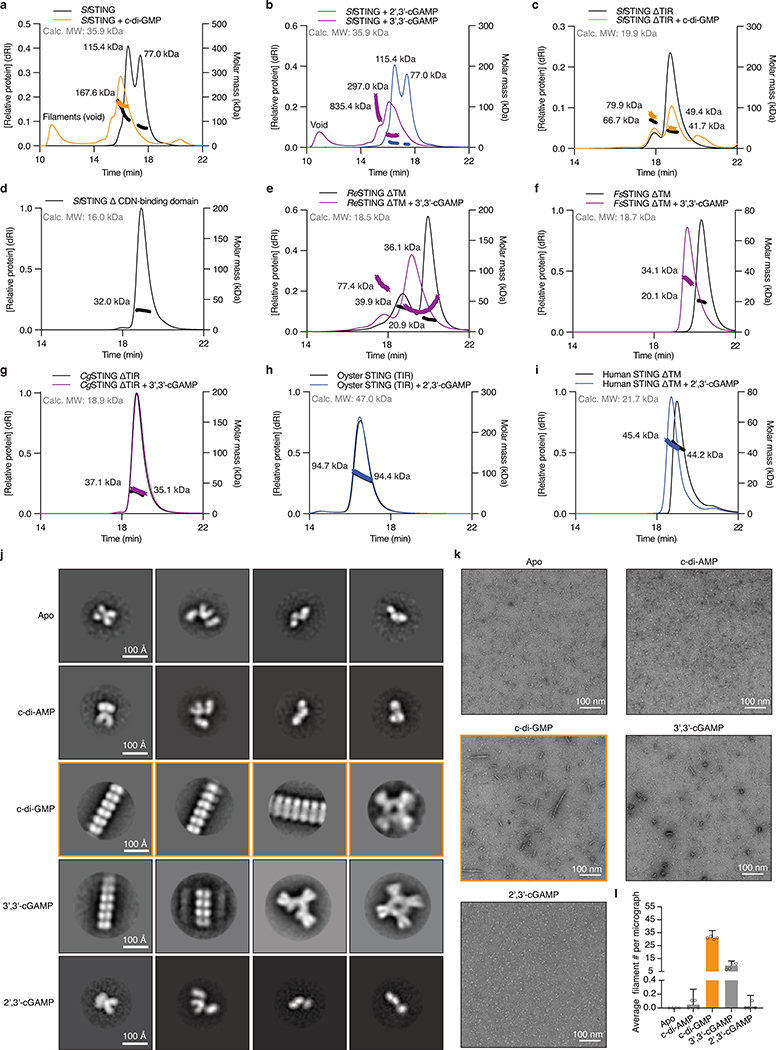
Extended Data Figure 7 |. Conservation of oligomerization as a mechanism of STING activation.
a–i, STING SEC-MALS analysis. a,b, Full-length SfSTING changes oligomeric state in the presence of c-di-GMP or the weak agonist 3′,3′-cGAMP, and does not change oligomeric state in the presence of 2′,3′-cGAMP. c, With the TIR domain removed (ΔTIR), SfSTING no longer forms higher order complexes but notably remains dimeric in the apoprotein form. Bacterial TIR-STING proteins therefore appear to require the TIR domain to maintain stable higher-order oligomerization suggesting that intermolecular contacts are made with both TIR and STING domains. d, A TIR-only construct of SfSTING with the cyclic dinucleotide binding domain removed (ΔCDN) elutes as a single species which is consistent with the molecular weight for a homodimer. h, human STING (ΔTM) is a dimer in solution with or without 2′,3′-cGAMP, confirming that TM contacts are required for oligomerization and filament formation12,23. Nearly all tested bacterial and metazoan STING constructs migrate as dimers in solution consistent with the cyclic dinucleotide binding domain forming a constitutive homodimeric complex for ligand recognition. Two exceptions include ReSTING (ΔTM) and FsSTING (ΔTM) which form a mixture of monomeric and dimeric states in the absence of ligand and dimers or tetramers in the presence of 3′,3′-cGAMP. These results indicate that alternative oligomerization events may be required for activation of bacterial TM-STING effector function.
j, Negative stain electron microscopy 2D class averages for SfSTING (E84A mutant) alone or in the presence of cyclic dinucleotide ligands. Stable STING filament formation requires c-di-GMP. 2D class averages were derived from particles selected from 75 micrographs for each condition.
k, Representative micrograph images reveal extensive filament formation of varying length and orientation in the presence of c-di-GMP. Apo, c-di-AMP, and 2′,3′-cGAMP micrographs lack filaments. Images are each representative of n = 75 micrographs for each condition.
l, Particle counting analysis of micrograph images shows that c-di-GMP induces more filament formation than 3′,3′-cGAMP and stable filament formation does not occur in the presence of c-di-AMP or 2′,3′-cGAMP. Data are mean ± s.d. for quantification of n = 4 groups of 10 micrograph images each.