Figure 6:
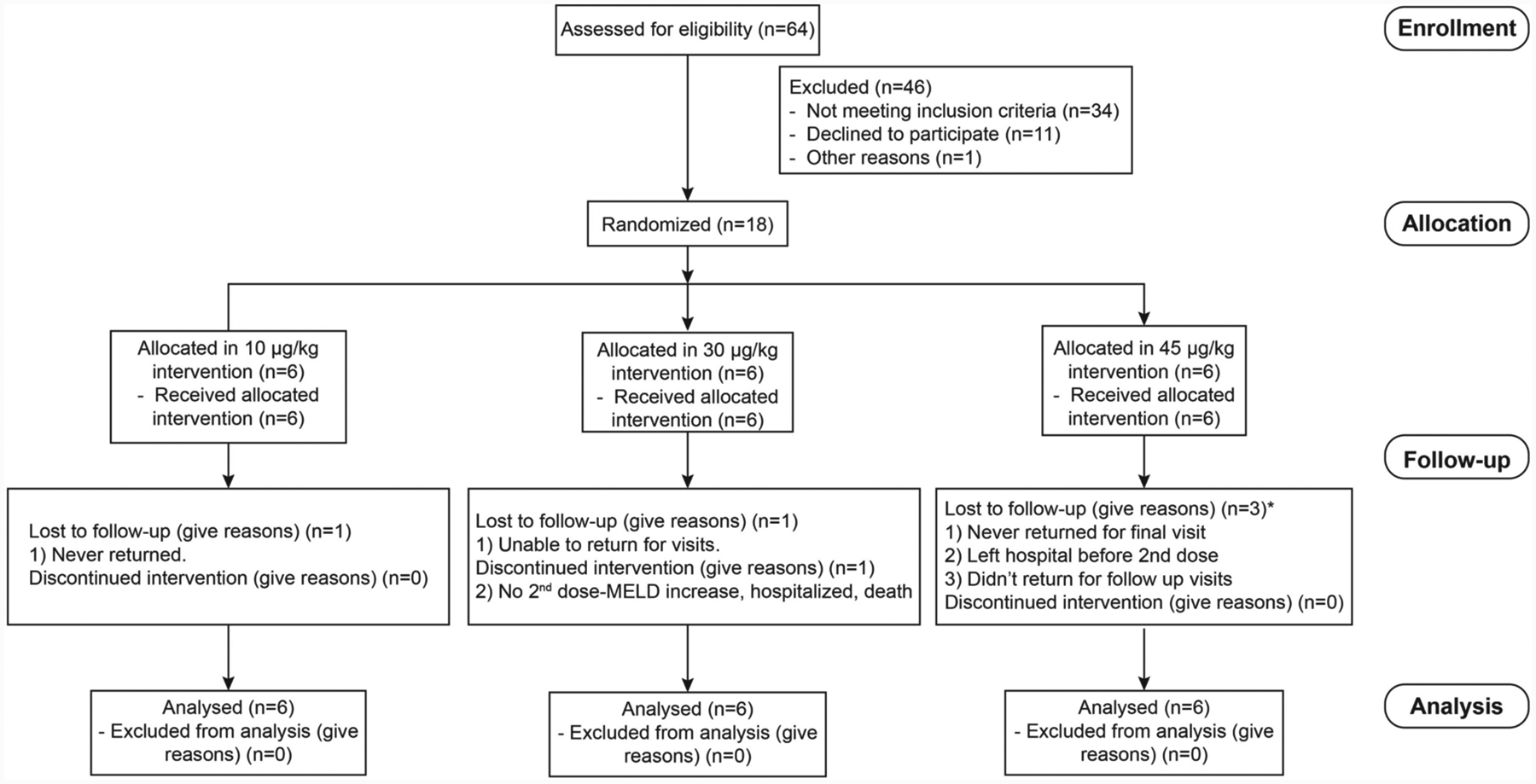
Consolidated Standards of Reporting Trials (CONSORT) flowchart from enrolled patients. An open-label, cohort dose-escalation phase Ha study to assess the safety and efficacy of IL-22 in patients with moderate and severe AH was performed. In detail, 18 patients (9 moderate and 9 severe AH) were enrolled, and three doses of IL-22Fc (10, 30, 45 μg/kg) were administered. Adapted from Arab et al.: Hepatology 2019 Nov 27. doi: 10.1002/hep.31046
