Figure 2. mAb two-step binding to rPfCSP is associated with high junctional affinity.
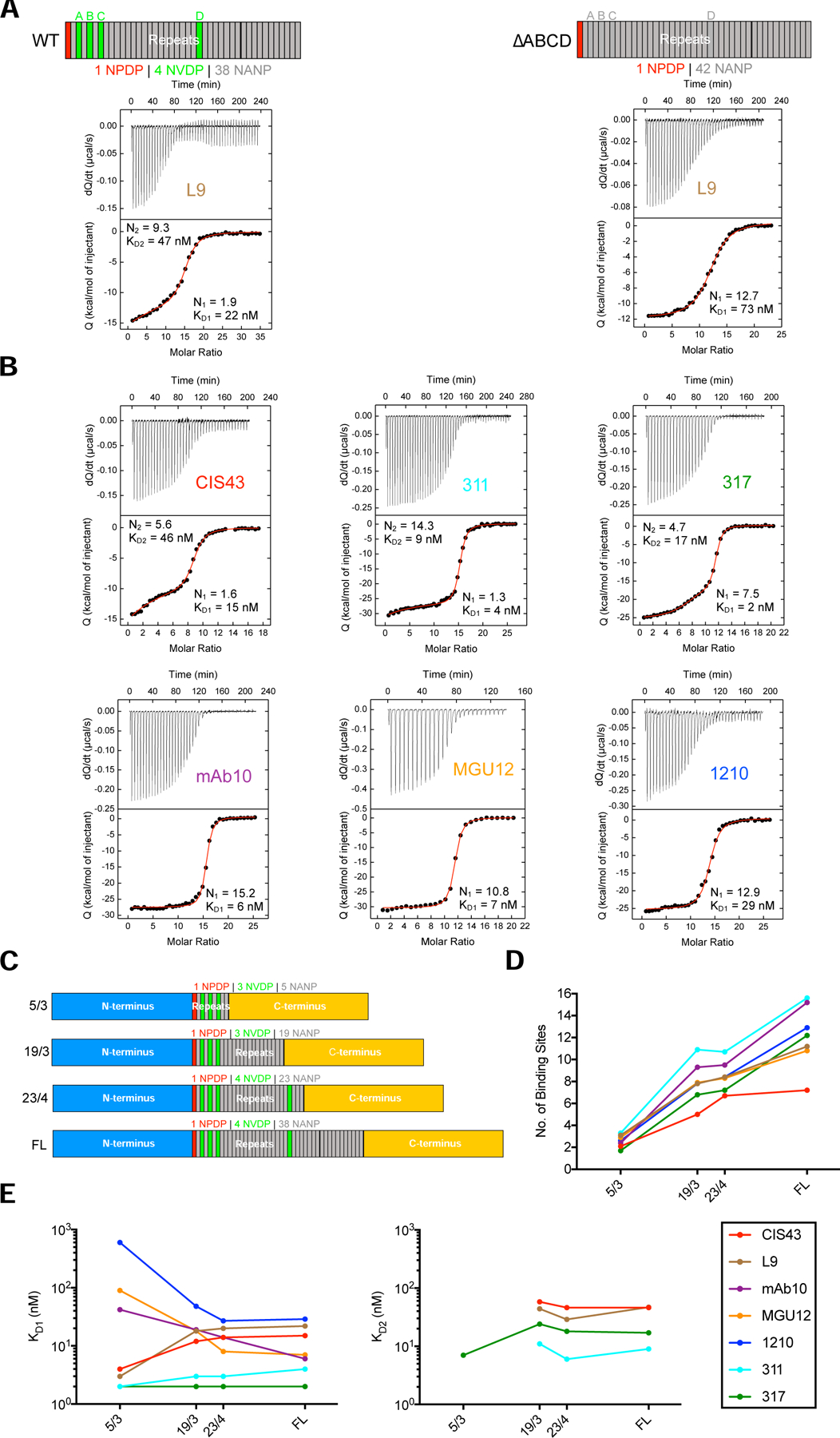
(A) ITC analyses of L9 IgG binding to wild-type rPfCSP (rPfCSP_WT or FL) and rPfCSP with all four NVDP mutated to NANP (rPfCSP_∆ABCD); N- and C-termini in schematics were omitted. Top, dQ/dt (heat flow, Q, as a function of time). Bottom, the integrated heat associated with each IgG injection shown as a function of the molar ratio between IgG antigen binding sites and rPfCSP in the calorimetric cell. The red line represents the result from best nonlinear least squares fit of the data. Dissociation constant (KD) and stoichiometry (N) of binding are shown for binding events 1 and 2. ITC data was fit to a two-step binding model if the IgG titrant bound to two sets of sites with different affinity values. The first set of high-affinity sites is saturated at lower IgG concentrations before the second set of lower-affinity sites. (B) ITC analyses of CIS43, 311, 317, 1210, mAb10, and MGU12 IgG binding to rPfCSP_WT (or FL). (C) Schematics of rPfCSP_FL and rPfCSP mutants with truncated repeat regions (23/4, 19/3, 5/3 NANP/NVDP repeats) and identical N- and C-termini. (D) Aggregate stoichiometry (binding events 1+2; no. of antigen binding sites) of mAb binding to 5/3, 19/3, 23/4, and rPfCSP_FL determined through ITC. (E) Affinity (binding events 1 vs. 2; KD, nM) of mAb binding to 5/3, 19/3, 23/4, and rPfCSP_FL determined through ITC. All ITC plots are representative of 2–3 independent experiments; D-E reflect an average of these experiments. See also Figures S1–2; Tables S1–2, S4.
