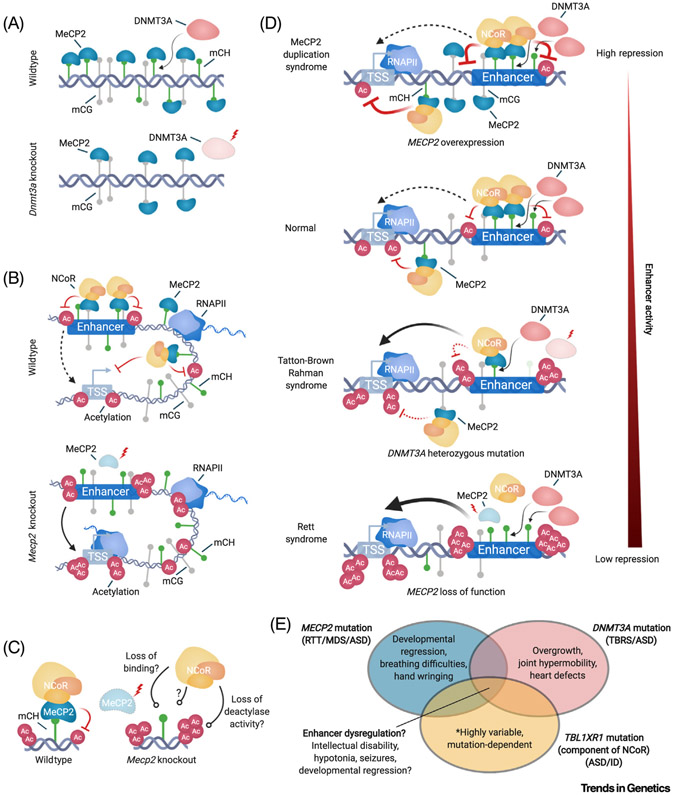
Figure 3. Molecular Mechanisms of the mC-MeCP2-NCoR Axis and Its Disruption in Neurodevelopmental Disorders.
(A) Top, a model depicting that MeCP2 binds to mCH deposited by DNMT3A. Bottom, upon complete or conditional knockout of Dnmt3a, mCH binding sites for MeCP2 are lost, but binding of MeCP2 to mCG persists. (B) Top, model of MeCP2 binding to mC at enhancers and gene bodies to reduce the acetylation of enhancers, genes, and promoters, resulting in a reduction of transcription initiation. Bottom, upon Mecp2 knockout, restriction on acetylation and transcription are reduced. (C) Left, the MeCP2-NCoR complex bound to mC represses enhancer acetylation. This may occur directly through the HDAC component of the complex or indirectly through other undefined activities of NCoR. Right, loss of MeCP2 can lead to loss of NCoR recruitment to the genome, loss of co-repressor activities that result in reduced acetylation, or potentially a mechanism yet to be defined. (D) Schematic depicting a spectrum of disruption for MeCP2 repressive effects at enhancers across neurodevelopmental disorders; ranging from hyper repression in MeCP2 duplication, to intermediate disruption of repression occurring when heterozygous loss of DNMT3A leads to global reduction in mCH, to complete loss of repression when MeCP2 is knocked out. (E) Venn diagram illustrating mechanistic and phenotypic overlaps of molecular pathologies of neurological disorders. Disruption of enhancer repression by the mC-MeCP2-NCoR axis may be shared across these disorders and contribute to pathology. Notably, each genetic lesion results in unique phenotypes that are likely to drive loss of molecular functions outside of the overlapping mC-MeCP2-NCoR axis. Abbreviations: ASD, Autism spectrum disorder; DNMT3A, DNA methyltransferase 3A; ID, intellectual disability; MDS, MeCP2 duplication syndrome; MeCP2, methyl CpG-binding protein 2; NCoR, nuclear co-repressor complex; RNAPII, RNA polymerase II; RTT, Rett syndrome; TBRS, Tatton-Brown Rahman syndrome; TSS, transcription start site.