Abstract
Cutaneous melanomas may demonstrate a variety of histopathological features and genetic abnormalities. Melanomas that arise in the setting of blue nevi, also known as “malignant blue nevus” or melanoma ex blue nevus (MBN), share similar histopathological and mutational profile with uveal melanoma. The majority of uveal melanomas show characteristic GNA11 or GNAQ mutations; additional BAP1 mutation or loss is associated with the highest risk for metastasis and worst prognosis. However, the significance of BAP1 loss in melanomas ex blue nevus remains unclear. We present a case of melanoma ex blue nevus arising from the scalp of a twenty-one-year-old female. The diagnosis was established on histopathological findings demonstrating a markedly atypical melanocytic proliferation with increased mitotic activity, necrosis, and a focus of angiolymphatic invasion. Immunohistochemical analysis demonstrated the absence of BAP1 nuclear expression within tumor cells. Next Generation Sequencing detected GNA11 Q209L mutation and BAP1 loss (chromosome 3p region loss), supporting the diagnosis. We reviewed another twenty-one MBN cases with reported BAP1 status from the literature. MBN with BAP1 loss presented at a younger average age (41 years versus 61 years), demonstrated larger average lesion thickness (9.0 mm versus 7.3 mm), and had a higher rate of metastasis (50% versus 33%) compared with BAP1-retained MBN. BAP1 expression studies may assist in the diagnosis and management of MBN, but further research is needed.
Keywords: melanoma ex blue nevus, malignant blue nevus, BAP1 loss, melanoma
Introduction
Blue nevi are a heterogeneous group of tumors that arise in the dermis from spindle and dendritic melanocytes. The majority of blue nevi are benign, but rarely malignant melanoma may show histologic features of a blue nevus or arise within a blue nevus.1 Melanomas ex blue nevus (also known as “malignant blue nevus”) tend to affect younger adults and are usually located on the scalp.2 Distinguishing them from benign lesions or metastatic melanoma may be challenging on histological grounds. We present a case of a melanoma ex blue nevus and discuss how Next Generation Sequencing (NGS) and BAP1 expression status may be helpful in the diagnosis and management of these lesions.
Case Report
A twenty-one-year-old female presented with a five-month history of a two-centimeter blue/black subcutaneous nodule on the scalp (Fig. 1). The lesion had been gradually enlarging and becoming tender. The patient denied family history of melanoma, mesothelioma or renal cell carcinoma. A biopsy from the lesion revealed a broad and poorly demarcated intradermal proliferation composed of sheets and infiltrative fascicles of markedly atypical melanocytes that were epithelioid to fusiform in shape (Fig. 2A). These cells had enlarged, pleomorphic, vesicular nuclei, prominent nucleoli, and ample cytoplasm with dusty melanin (Fig. 2B). Scattered background melanophages with heavy pigmentation, areas of rhabdoid cytological features and conspicuous increased mitotic activity (3 mitosis/mm2) were noted. Tumor cells were diffusely positive for Sox-10 and Melan-A (Fig. 2C). Ki-67 stain demonstrated a high proliferative index. Based on these findings, a diagnosis of primary or metastatic malignant melanoma was rendered. To further classify the nature of the lesion, NGS was performed and revealed GNA11 Q209L mutation, loss of short arm of chromosome 3 (3p) that harbors BAP1 gene, and copy number gain of chromosome 8q region harboring the MYC gene. Immunohistochemical analysis confirmed the loss of BAP1 nuclear expression within the tumor cells (Fig. 2D). EWSR1 fluorescence in situ hybridization (FISH) study was performed to exclude clear cell sarcoma and was negative for rearrangement. Based on these molecular and immunohistochemistry findings, a diagnosis of a melanoma ex blue nevus with BAP1 loss was established.
Figure 1.
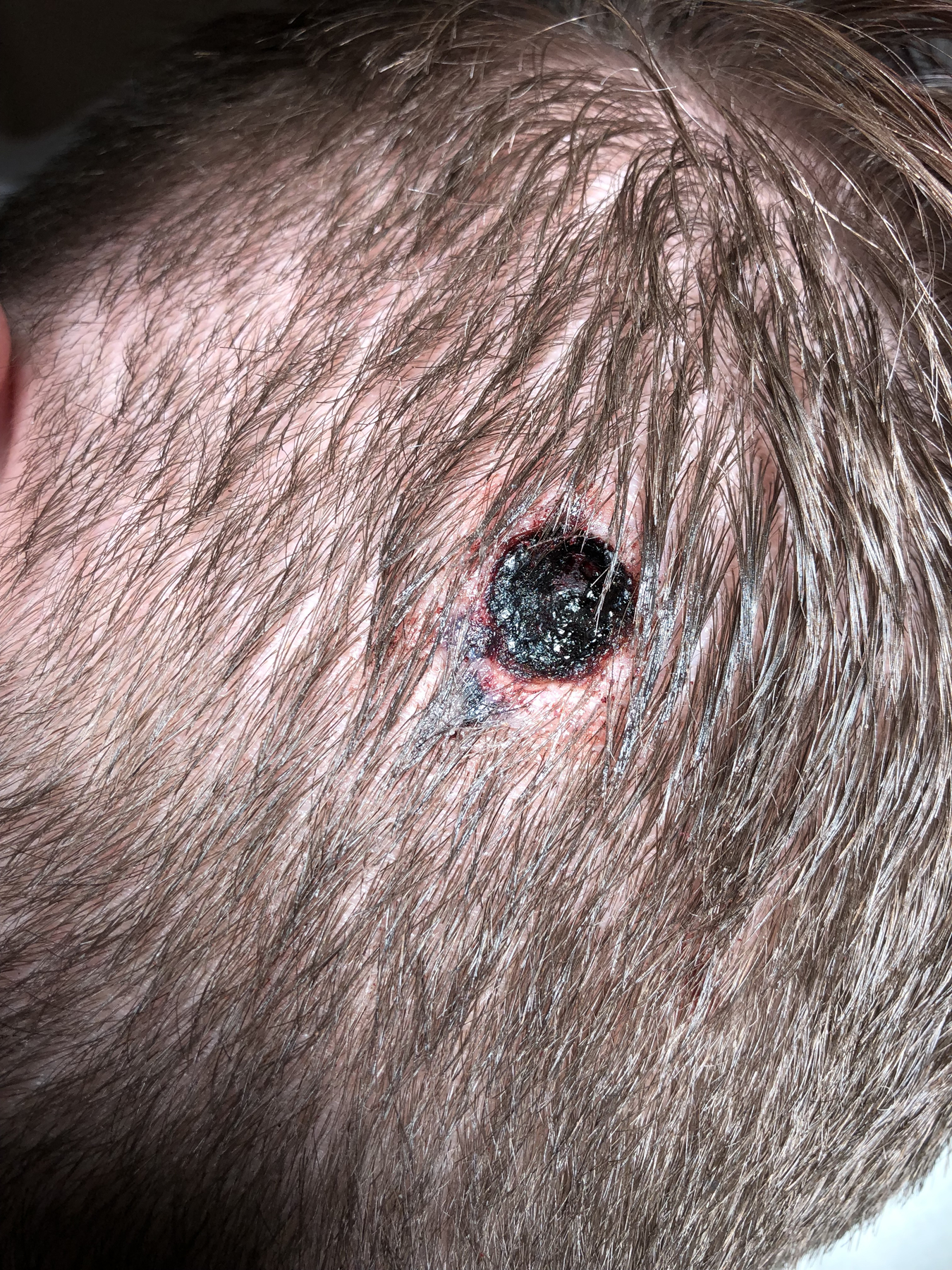
Image taken after the biopsy of the lesion. Initial presentation revealed a two-centimeter blue/black subcutaneous nodule on the left occipital scalp of a twenty-one year-old female.
Figure 2.
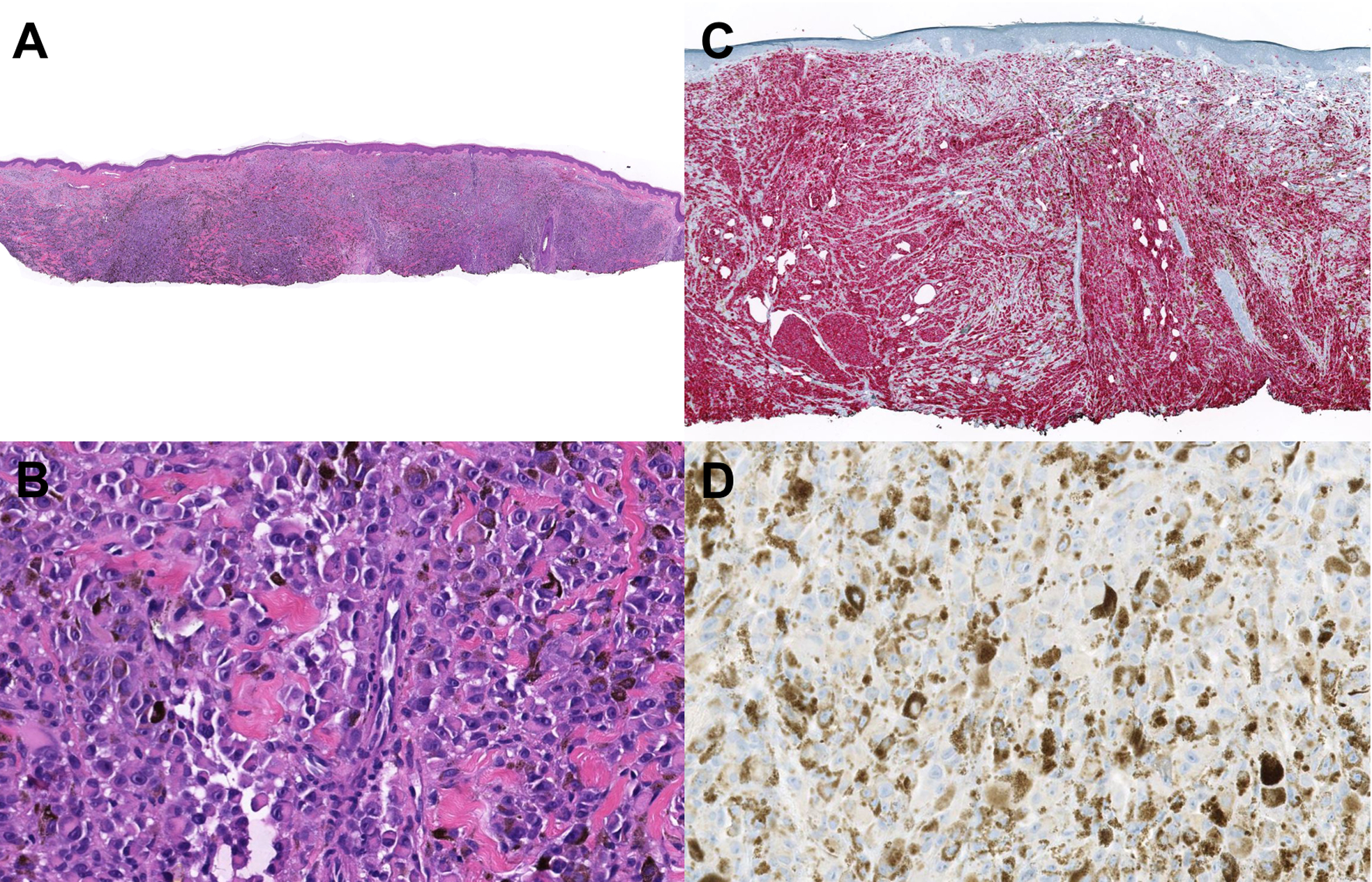
Histolopathologic examination of the biopsied lesion. (A) Scanned and cropped H&E slide showing a melanocytic lesion with sheet-like atypical melanocytes and heavy pigmentation. (B) Scanned and cropped H&E slide with a higher magnification demonstrating atypical melanocytes with a rhabdoid morphology. (C) Melan-A immunostaining showing diffuse expression of Melan-A immunostain in the tumor. (D) BAP1 immunostaining showing loss of nuclear BAP1 expression in melanoma cells.
The patient underwent wide local excision of the lesion with two-centimeter margins. The excision specimen demonstrated an invasive atypical intradermal melanocytic tumor with a maximum thickness of 9.5 millimeters (Fig. 3). Large areas of necrosis, atypical cell morphology (rhabdoid, epithelioid, fusiform), increased mitotic activity and focal angiolymphatic invasion were seen in the re-excision specimen. Computed tomography (CT) of chest, abdomen and pelvis, and magnetic resonance imaging (MRI) of the brain revealed no regional or distant metastases. Dilated ophthalmic exam was negative for choroidal lesions. Dissection of three left cervical draining lymph nodes revealed no metastatic disease. Follow up brain and neck MRI eight months after the initial diagnosis was negative for metastatic disease. No evidence of recurrence was detected ten months after the wide local excision.
Figure 3.
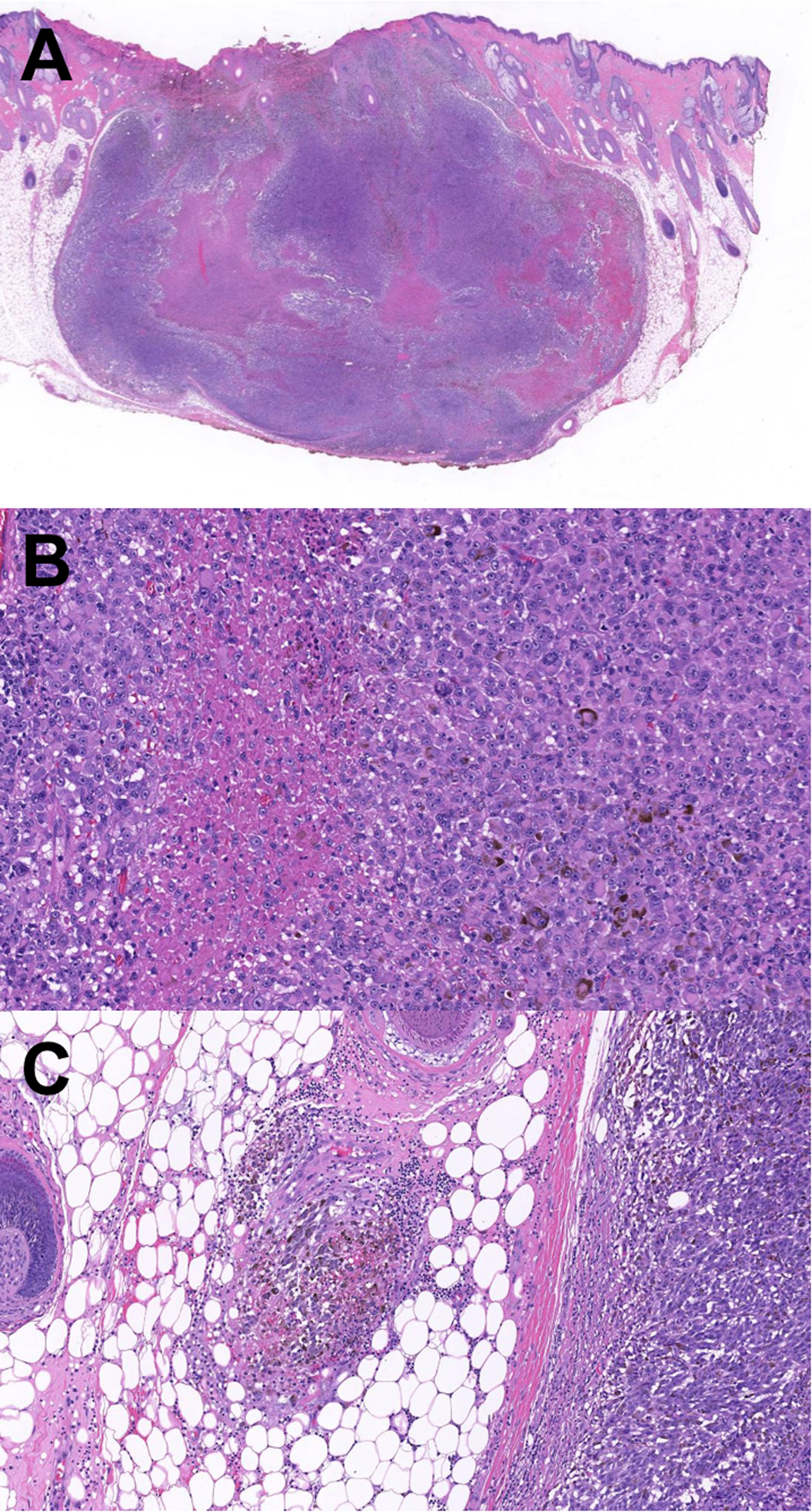
Histopathologic examination of the re-excision specimen. (A) Re-excision of the neoplasm demonstrating a large nodule with extensive necrotic areas. H&E stain, scanned and cropped image. (B) Higher magnification shows necrotic areas and sheets of highly atypical pigmented melanocytes. H&E stain, scanned and cropped image. (C) A destroyed vessel with angioinvasion at the vicinity of the melanoma. H&E stain, scanned and cropped image.
Discussion
The term “malignant blue nevus” was first used in 1953 to describe malignant melanoma arising within a cellular blue nevus.1 Since then, other terms such as “melanoma associated with blue nevus,” “blue nevus-like melanoma” and “melanoma ex blue nevus” have been used to imply this entity.
Histopathologically, melanoma ex blue nevus may be difficult to distinguish from metastatic melanoma, pigmented epithelioid melanocytoma, and atypical spitzoid or atypical deep penetrating melanocytic tumors.3 However, it is essential to classify melanocytic lesions accurately for correct clinical management. A case series of thirty-three melanocytic lesions with features of blue nevi demonstrated that, notwithstanding their morphology, some lesions harbored BRAFV600E mutation and would have been better classified as atypical common nevi or conventional melanomas.4
Genetically, blue nevi and melanomas ex blue nevus are distinct from epidermis-derived common acquired nevi or “conventional” malignant melanoma, and are more closely related to uveal melanoma. Common acquired nevi and melanomas frequently harbor BRAF or NRAS mutations, but the majority of blue nevi carry GNAQ or GNA11 mutations, similar to uveal melanoma.5 GNAQ and GNA11 are components of G proteins, and mutations in these genes activate the downstream mitogen-activated protein kinase (MAPK) pathway, leading to melanocytic proliferation. BRAF and NRAS mutations also activate the MAPK pathway. Mutations in BRAF/NRAS and GNAQ/GNA11 have been shown to be mutually exclusive in melanocytic lesions.6
In uveal melanoma, loss of BAP1 (BRCA1 associated protein 1), a tumor suppressor gene, due to a mutation or deletion or loss of chromosome 3, is associated with the highest rate of metastasis and worst prognosis. Germline mutations in BAP1 have been identified in familial syndromes with an increased incidence of uveal melanoma, cutaneous melanoma, mesothelioma, renal cell carcinoma, and atypical melanocytic tumors, known as BAP1 inactivated melanocytic tumor.
Little is known about the significance of BAP1 loss in melanomas ex blue nevus. A previous study showed that the loss of chromosome 3p region was detected in three of nine melanoma ex blue nevus, but in none of the seventeen blue nevi cases.7 In another study, the loss of BAP1 expression was found exclusively in malignant but not in benign counterparts of this blue nevi family. Three melanoma ex blue nevus that developed distal metastases all demonstrated BAP1 loss.2 The authors predict that melanomas ex blue nevus with BAP1 loss may have aggressive behavior and greater metastatic potential, similar to uveal melanoma.
We reviewed an additional twenty-one melanoma ex blue nevus cases previously published in the literature with known BAP1 status (Table 1). In these twenty-two cases (including our case), the average age at presentation of tumors with and without BAP1 loss was 41 versus 61 years. The average tumor thickness of melanomas with and without BAP1 loss was 9.0 mm versus 7.3 mm. The incidence of metastases developed in melanomas with and without BAP1 loss were 50% (5/10) versus 33% (3/9). The time to the development of metastases ranged from 19 to 144 months. In uveal melanoma, one early study showed that 57% of thirty patients with the loss of one chromosome 3 region relapsed with metastatic disease; none of the twenty-four patients without the chromosome 3 loss developed metastatic disease.8 These data suggest that loss of BAP1 expression in melanoma ex blue nevus may be an important prognostic factor with potential for metastasis similar to uveal melanoma. In addition, BAP1 loss by immunohistochemistry is a very useful diagnostic marker while dealing with atypical blue nevi tumors. These results deserve further validation and investigation using larger cohorts.
Table 1.
Twenty-two published malignant blue nevi cases that have available BAP1 expression status based on immunohistochemical staining. Nineteen of these cases have clinical follow-up data.
| Case | Age | Sex | Site | Follow-up (mo) | Outcome | Metastasis | Thickness (mm) | BAP1 Status | GNA11/GNAQ Mutation | |
|---|---|---|---|---|---|---|---|---|---|---|
| Yeh 201413 | 1 | 64 | F | Scalp | 22 | No evolution of disease | None | Loss | Not tested | |
| Dai 201614 | 1 | 46 | F | Scalp | 72 | Died from complications of sepsis | Liver metastasis | Loss | GNA11 | |
| Costa 20162 | 1 | 21 | M | Scalp | 19 | Died of disease | Liver metastasis | 5 | Loss | GNA11 |
| 2 | 26 | M | Scalp | 7 | Loss | GNA11 | ||||
| 3 | 27 | F | Shoulder | 74 | Died of disease | Widespread metastasis | 7 | Loss | GNA11 | |
| 4 | 36 | F | Scalp | 18 | No evolution of disease | Cervical lymph node metastasis | 13 | Loss | GNA11 | |
| 5 | 49 | M | Scalp | 6 | No evolution of disease | None | 7 | Loss | GNA11 | |
| 6 | 49 | F | Scalp | 29 | Stable disease | None | 14 | Loss | WT | |
| 7 | 82 | F | Scalp | 18 | No evolution of disease | None | 9.2 | Loss | WT | |
| 8 | 56 | M | Scalp | 23 | No evolution of disease | None | 20 | Normal | GNA11 | |
| 9 | 69 | F | Scalp | 3 | No evolution of disease | None | 6 | Normal | GNAQ | |
| 10 | 75 | F | Scalp | 24 | No evolution of disease | None | 8 | Normal | GNA11 | |
| 11 | 79 | F | Scalp | 4 | No evolution of disease | None | 7.4 | Normal | GNA11 | |
| Griewank 20174 | 22 | 88 | M | Scalp | 30 | Died of Alzheimer’s, HF and metastatic disease | Metastatic disease | Normal | WT | |
| 23 | 61 | M | Scalp | 72 | Died of disease | Metastatic disease | Normal | GNA11 | ||
| 26 | 35 | M | Gluteal region | 13 | No evolution of disease | None | 6 | Normal | GNAQ | |
| 27 | 39 | M | Scrotum | 4 | No evolution of disease | None | Normal | GNA11 | ||
| 36 | 64 | M | Temporal | 144 | Cervical lymph node metastasis | Neck soft tissue metastasis | 7.5 | Normal | GNA11 | |
| 37 | 66 | M | Pectoral | 1.45 | Normal | GNA11 | ||||
| 38 | 31 | M | Temporal | 103 | Progressive disease | Widespread disease | Loss | GNA11 | ||
| Castillo 201815 | 1 | 39 | M | Scalp | 2.1 | Normal | GNAQ | |||
| This report | 1 | 21 | F | Scalp | 10 | No evolution of disease | None | 9.5 | Loss | GNA11 |
Very few studies have compared clinical outcomes of conventional melanomas and melanoma ex blue nevus with similar disease burden. It remains unclear whether the prognostic factors for conventional cutaneous melanoma are applicable to melanomas with GNAQ/GNA11 mutations. One study that reviewed ten melanoma ex blue nevus cases suggested these lesions were highly aggressive tumors with a propensity for metastasis.9 Further studies compared cohorts of conventional and GNAQ/GNA11 mutated melanomas with similar tumor burden showed despite presenting at an advanced stage, the clinical course and survival rate of melanoma ex blue nevus resembled those of conventional melanoma.10,11 Another study analyzed twenty-one melanoma ex blue nevus cases and found a significant correlation between tumor thickness and recurrence-free survival; other clinicopathological parameters such as anatomic location, lymphovascular invasion and perineural invasion did not show significant prognostic association.12 These results suggest that melanoma ex blue nevus should be managed similarly to conventional melanoma, but this recommendation needs to be validated by larger cohorts.
In conclusion, we herein describe a case of melanoma ex blue nevus arising from the scalp of a young female harboring GNA11 mutation and loss of BAP1 expression. The malignant nature of this melanocytic tumor was established based on marked histological atypia, high mitotic and proliferation index, large areas of necrosis and angiolymphatic invasion. NGS and BAP1 expression studies assisted in the further classification of the disease and subsequently appropriate management. BAP1 expression may have an important implication on the prognosis of this family of malignant lesions. However, further studies on larger cohorts are necessary to validate these results.
Footnotes
Conflict of interest: none
References
- 1.Allen AC, Spitz S. Malignant melanoma; a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer 1953; 6:1–45. [DOI] [PubMed] [Google Scholar]
- 2.Costa S, Byrne M, Pissaloux D, et al. Melanomas associated with blue nevi or mimicking cellular blue nevi: clinical, pathologic, and molecular study of 11 cases displaying a high frequency of GNA11 mutations, BAP1 expression loss, and a predilection for the scalp. Am J Surg Pathol 2016; 40:368–77. [DOI] [PubMed] [Google Scholar]
- 3.Magro CM, Crowson AN, Mihm MC. Unusual variants of malignant melanoma. Mod Pathol 2006; 19 Suppl 2:S41–70. [DOI] [PubMed] [Google Scholar]
- 4.Griewank KG, Müller H, Jackett LA, et al. SF3B1 and BAP1 mutations in blue nevus-like melanoma. Mod Pathol 2017; 30:928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009; 457:599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas RK, Baker AC, Debiasi RM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet 2007; 39:347–51. [DOI] [PubMed] [Google Scholar]
- 7.Chan MP, Andea AA, Harms PW, et al. Genomic copy number analysis of a spectrum of blue nevi identifies recurrent aberrations of entire chromosomal arms in melanoma ex blue nevus. Mod Pathol 2016; 29. doi: 10.1038/modpathol.2015.153. [DOI] [PubMed] [Google Scholar]
- 8.Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet 1996; 347:1222–5. [DOI] [PubMed] [Google Scholar]
- 9.Granter SR, McKee PH, Calonje E, et al. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called “malignant blue nevus”. Am J Surg Pathol 2001; 25:316–23. [DOI] [PubMed] [Google Scholar]
- 10.Kachare SD, Agle SC, Englert ZP, et al. Malignant blue nevus: clinicopathologically similar to melanoma. Am Surg 2013; 79:651–6. [PubMed] [Google Scholar]
- 11.Martin RCW, Murali R, Scolyer RA, et al. So-called “malignant blue nevus”: a clinicopathologic study of 23 patients. Cancer 2009; 115:2949–55. [DOI] [PubMed] [Google Scholar]
- 12.Loghavi S, Curry JL, Torres-Cabala CA, et al. Melanoma arising in association with blue nevus: a clinical and pathologic study of 24 cases and comprehensive review of the literature. Mod Pathol 2014; 27:1468–78. [DOI] [PubMed] [Google Scholar]
- 13.Yeh I, Mully TW, Wiesner T, et al. Ambiguous Melanocytic Tumors With Loss of 3p21. Am J Surg Pathol 2014. doi: 10.1097/PAS.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai J, Tetzlaff MT, Schuchter LM, et al. Histopathologic and mutational analysis of a case of blue nevus-like melanoma. J Cutan Pathol 2016; 43:776–80. [DOI] [PubMed] [Google Scholar]
- 15.Castillo SA, Pham AK, Barton DT, et al. A diagnostically-challenging case of melanoma ex blue nevus with comprehensive molecular analysis, including the 23-gene expression signature (myPath melanoma). J Cutan Pathol 2019; 46:226–30. [DOI] [PubMed] [Google Scholar]


