Figure 2.
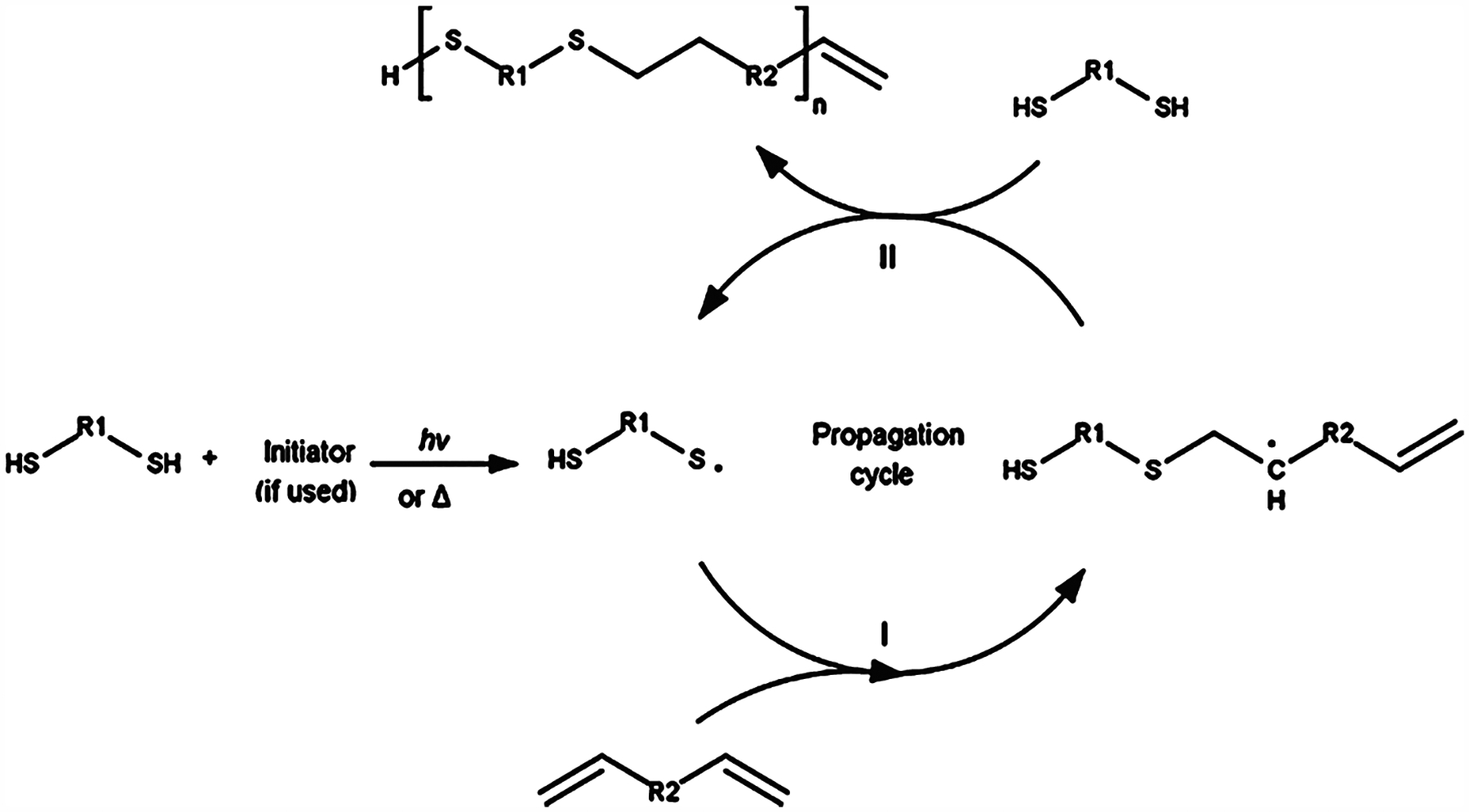
Free radical initiated thiol–ene click chemistry reaction mechanism. Propagation occurs in mechanism I. The initiator free radical abstracts the thiol hydrogen, producing a thiyl radical that attacks the alkene double bond. Chain transfer occurs in mechanism II. The thiyl radical is regenerated by the alkyl radical abstracting a free thiol hydrogen, which under the right reaction conditions will occur much more often than attacking another alkene double bond. The thiyl radical can now continue to propagate the thiol–ene reaction. Reproduced with permission from ref 60. Copyright 2017 Elsevier.
