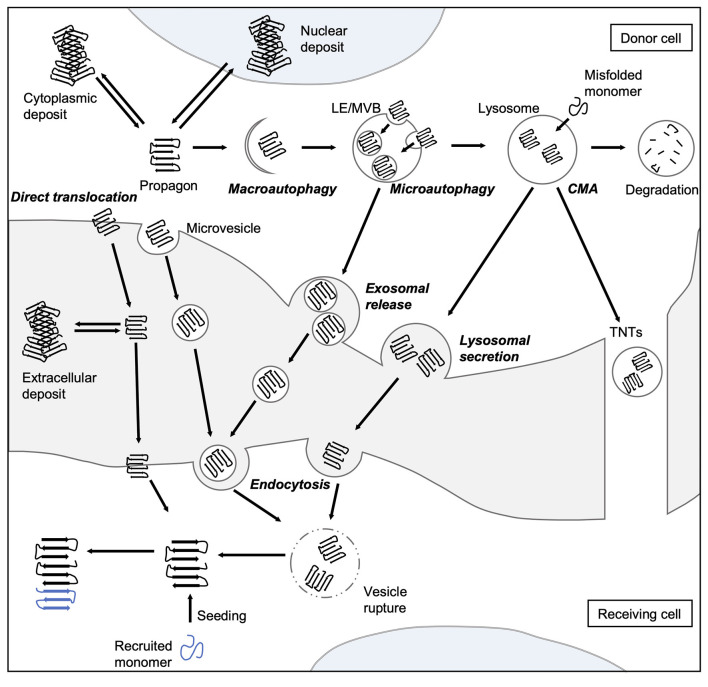
Figure 2.
Spreading routes of amyloidogenic proteins. The intercellular transmission of disease proteins can occur via several pathways. Some substrates can be translocated directly across the plasma membrane or shed via microvesicles. Misfolded proteins can be also targeted by all branches of autophagy, and thereby enter the endo-lysosomal system. Aggregates can either be engulfed via bulk or selective (involving adaptor proteins, such as sequestosome 1 (SQSTM1/p62) macroautophagy or taken up into LEs and MVBs through microautophagy. Abnormal proteins may also be directly ingested by lysosomes as a result of CMA. Degradation-resistant aggregates may be released into the extracellular space by lysosomal fusion with the plasma membrane or transported into neighboring cells via TNTs. Endocytosis mediates the uptake of either free protein or extracellular vesicles containing the disease-associated protein. To be released into the cytosol of the receiving cell, misfolded proteins can induce endocytic vesicle rupture. Released proteins can then recruit monomers and catalyze their incorporation, which eventually leads to the formation of amyloid aggregates in the receiving cell. CMA, chaperone-mediated autophagy; LE, late endosome; MVB, multivesicular body; TNT, tunneling nanotube.