Figure 3.
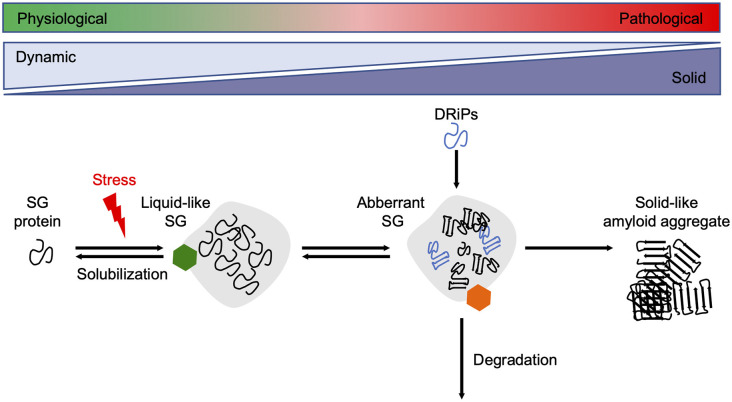
Chaperones maintain SG dynamics. Proteins containing intrinsically disordered domains can undergo phase separation upon cellular stress, such as heat stress, and form liquid-like SGs. Under healthy conditions, the SGs are dissolved when the stress subsides. Chaperones (green hexamer) such as Hsp70s, sHsps, and DNAJB6 are involved in the resolubilization process and thus regulate SG homeostasis. Disease-associated mutations in SG associated proteins, the accumulation of DRiPs, or prolonged exposure to stress conditions reduce the fluidity of SGs, leading to a more solid-like structure that promotes amyloid formation over time. The HSPA1A-BAG3-HSPB8 chaperone network (orange hexamer) targets aberrant SGs for degradation. SG, stress granule.